Chemist proposes new method for green synthesis of xanthene derivatives
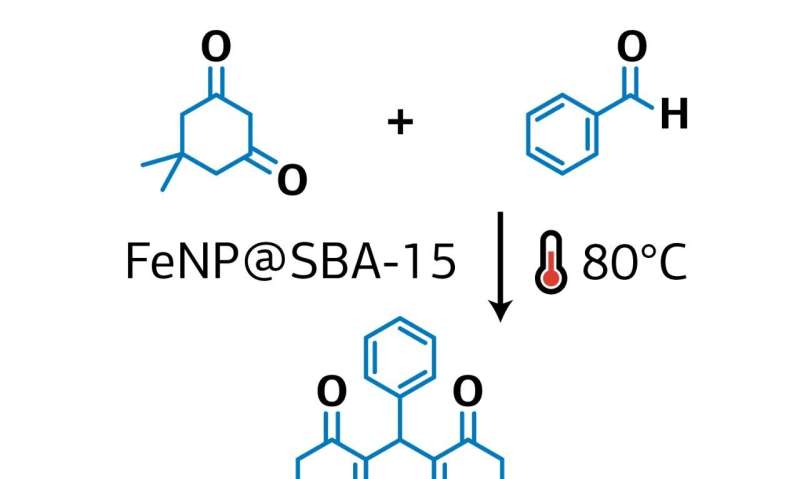
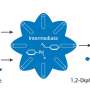
A RUDN chemist has proposed a new method for the synthesis of xanthene derivatives, which are used as the basis for drug manufacturing—from antidepressants to antiviral and antitumor drugs. The method is based on the example of the synthesis of 1,8-dioxoctahydroxantene and its derivatives. To that end, a safe and stable catalyst based on iron oxide was created on the surface of porous silicon oxide. As a result of the reaction, the yield of xanthene derivatives reached 99 percent. The results were published in Materials.
Due to their biological activity, xantenes and their derivatives, heterocycles of the dibenzopyran group, are used in the preparation of antidepressants, antimalarial, anti-inflammatory, antiviral and antibacterial agents, as well as in the development of photosensitizers in photodynamic therapy. In the synthesis of xanthene, a wide range of catalysts is used, from organic acids to porous silicon dioxide. However, in most cases, the catalysts have several disadvantages: They require the use of toxic or expensive agents, have a long reaction time, low thermal stability and low yields, and cause the formation of undesirable or toxic byproducts. Therefore, researchers seek more effective, practical and useful methods for producing xanthenes and their derivatives.
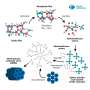
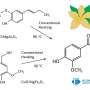
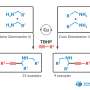
Rafael Luque from RUDN University and his colleagues have developed a new environmentally friendly and economical method for producing xanthenes using 1,8-dioxoctahydroxantene and its derivatives without the use of a solvent. The chemists used iron oxide nanoparticles deposited on the surface of porous silicon oxide as a catalyst. Xanthenes were obtained from aromatic aldehydes and dimedone, and the catalyst itself was developed by the authors in previous articles.
"The proposed method is simple, environmentally friendly, efficient and amenable for a large number of substrates. The catalyst has already been employed for the production of a large range of biologically active compounds and we plan to continue with additional efforts in the future," says Luque.
The authors investigated the catalytic activity of the new catalyst and its specific surface area. They also selected the optimal reaction conditions—temperature, time and amount of catalyst at which the highest yield of the target compound is observed.
The RUDN chemists found that the highest yield of 99 percent is achieved with small catalyst loads of about 0.1 percent molar at 80 °C for 30 minutes. In this case, the reaction proceeds best without the use of a solvent, and the catalyst itself practically does not lose its activity after 12-fold repeated use.
The authors of the paper emphasize the prospects of using the developed catalyst based on iron oxide as an environmentally friendly and economically viable alternative for the production of xanthene derivatives. Previous methods for the production of xanthene derivatives gave a lower yield within 86 to 93 percent, or they required the use of toxic catalysts.
More information: Fatemeh Rajabi et al. Solvent-Free Preparation of 1,8-Dioxo-Octahydroxanthenes Employing Iron Oxide Nanomaterials, Materials (2019). DOI: 10.3390/ma12152386
Provided by RUDN University