Chemist creates cheap catalysts for the production of vanillin
A chemist from RUDN University has created cheap and effective catalysts for the production of vanillin using spinel nanoparticles with copper oxide nanoparticles. Hydrogen peroxide was used as an oxidizing agent. The method allows the use of moderate temperatures and reduced reaction times. The paper was published in the journal Molecules.
Aromatic aldehydes are a class of organic compounds used in the food industry as flavorings. They are part of perfume compositions and also used in the production of drugs. One of the substances of this class is vanillin, which is in demand in both the food and perfume industries.
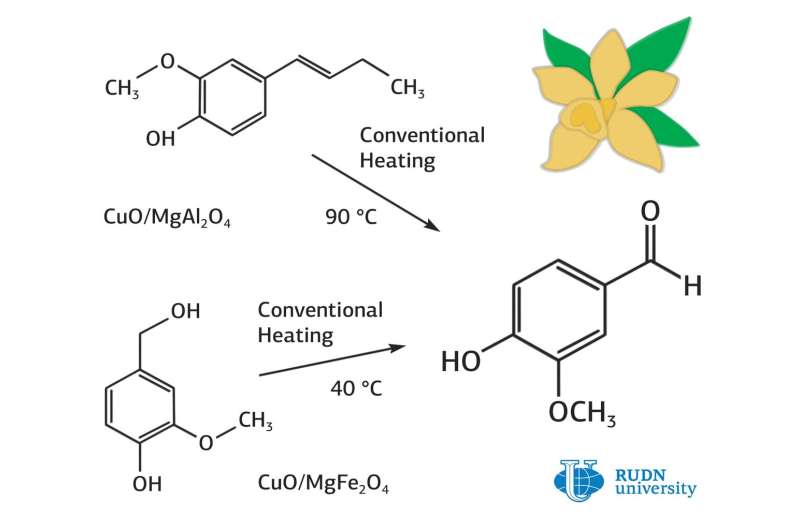
Currently, it is obtained by the oxidation of vanillin alcohol, but the catalysts for this reaction (palladium or gold) are expensive and require high temperatures (over 120 degrees Celsius) and long times for the reaction to be prepared and conducted. A chemist from RUDN University, Rafael Luque, and his colleagues have created catalysts that can lower the reaction temperature to 40 degrees Celsius if vanillin alcohol is used. The selectivity to obtain vanillin from vanillin alcohol was 100 percent.
The RUDN University chemist used two variants of the active component base: spinel nanoparticles based on aluminium and magnesium oxides, and iron and magnesium oxides. Professor Luque and his colleagues managed to improve the spinel synthesis method, which was previously more expensive, through the use of a high-speed ball mill. Copper oxide was used as an active component of the catalyst.
The oxidation reaction was carried out in acetonitrile. Hydrogen peroxide was used as an oxidizing agent. The progress of the reaction was monitored by gas chromatography. It turned out that the catalyst based on magnesium-aluminium spinel is more active. After eight hours of reaction, the conversion percentage of vanillin alcohol to vanillin was 81 percent. The selectivity to vanillin was 100 percent.
The catalysts can be used in the food, pharmaceutical, and perfume industries. They would help produce not only vanillin, but also other aromatic aldehydes.
More information: Behgam Rahmanivahid et al. Mechanochemical Synthesis of CuO/MgAl2O4 and MgFe2O4 Spinels for Vanillin Production from Isoeugenol and Vanillyl Alcohol, Molecules (2019). DOI: 10.3390/molecules24142597
Provided by RUDN University