Chemist creates efficient catalyst for organic sulfides synthesis
A RUDN chemist has obtained a new compound—a dumbbell-shaped phosphate-bridged molybdenum cluster. The cluster accelerates the reaction of the formation of sulfides from oxides and can be used in pharmaceutical and cosmetic manufacturing. The article was published in Inorganic Chemistry.
The reduction reaction of organic sulfides from oxides, or the deoxygenation of sulfoxides, is a simple direct process of the removal of oxygen from an organic oxide to obtain sulfide. This reaction is important because it happens in biological processes and in the synthesis of many organic compounds. Organic sulfides are present in most antibiotics, drugs and biological compounds. Sulfides are key intermediates in organic synthesis and are important components of many fine chemicals such as perfumes and cosmetics. Life forms use the enzyme with a molybdenum-containing cluster to accelerate sulfoxide reduction reactions. Molybdenum-based clusters are successfully used to produce not only synthetic sulfides, but also other classes of important organic compounds such as phosphines and olefins. The synthesis of new molybdenum catalysts with improved properties based on fundamental knowledge of deoxygenation mechanisms is an important and urgent task.
"A modern synthetic chemist is an architect and builder. Knowing the properties of building materials, the fragments of molecules, he first models the structure of a complex molecule, and then assembles it. Basing on the knowledge that phosphonic acids demonstrate a number of remarkable properties in the environment of molybdenum, we were able to obtain a dumbbell-type molybdenum cluster," write the authors.
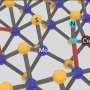
The molybdenum cluster was synthesized under hydrothermal conditions (in water and when heated) using the salts of molybdenum acid and a number of phosphonic acids. Phosphonic acids are used in many fields: the power industry, oil and gas production, oil refining, food, perfumery and textile industries. Phosphonic acids have a number of important properties for obtaining a dumbbell-shaped cluster. Firstly, they do not collapse when exposed to temperatures above 100 degrees Celsius; thus, they can be used in synthesis with a heat-up method. Secondly, they do not interact with water, therefore, in their synthesis, water can be used as a solvent. Thirdly, they have a high affinity for metal and a tendency to form bridge bonds. Using modern methods of modeling and structural analysis, it was possible to classify and precisely determine the structure of the new compound—the octanuclear molybdenum cluster, in which four molybdenum nuclei from below and four nuclei from above are linked by a phosphate bridge and are additionally stabilized by hydroxyl groups and ammonium ions.
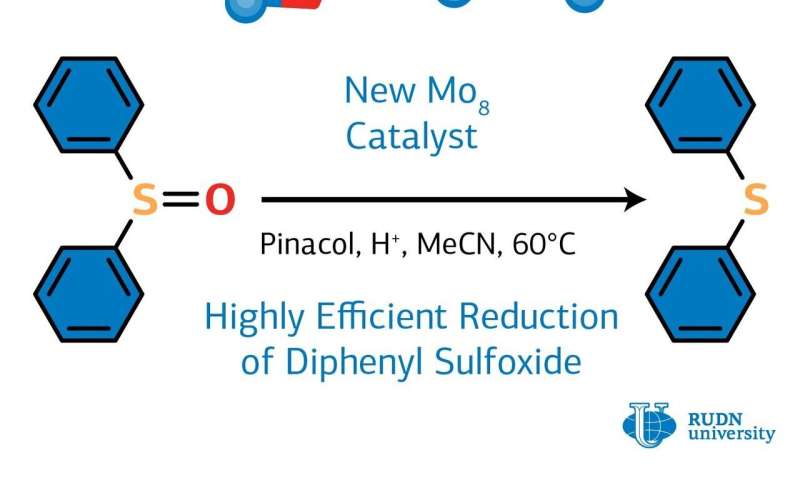
The resulting cluster was an efficient homogeneous catalyst for the reaction the reduction of diphenyl sulfoxide to diphenyl sulfide. A cheap and environmentally friendly pinacol reducing agent was used for the reaction. By varying synthesis conditions (duration, temperature, a type of solvent, an amount of catalyst), the chemists managed to achieve a yield of 99%. The cluster after heat treatment at 310 degrees Celsius showed another important property which is high proton conductivity. Thus, it can be used in the preparation of functional membrane materials for the creation of electrochemical devices such as sensors, fuel cells, and supercapacitors.
"After such a remarkable result in the diphenyl sulfide reduction reaction, we tested the catalyst in the reduction of other complex organic sulfides. And we were pleasantly surprised as the yields of the corresponding oxides were high as well. The catalyst can be used in the synthesis of an even greater number of different substances. In the future, we plan to test this catalyst in other catalytic transformations," the authors conclude.
More information: Eirini Armakola et al. Phosphonate Decomposition-Induced Polyoxomolybdate Dumbbell-Type Cluster Formation: Structural Analysis, Proton Conduction, and Catalytic Sulfoxide Reduction, Inorganic Chemistry (2019). DOI: 10.1021/acs.inorgchem.9b01376
Journal information: Inorganic Chemistry
Provided by RUDN University