An eco-friendly method for the synthesis of cinnamaldehyde
A RUDN University chemist has developed an ecologically safe method of obtaining cinnamaldehyde—a compound with antibacterial and anticancer activity. The scientist used catalysts based on iron and palladium nanoparticles to avoid the formation of environmentally harmful byproducts. This eco-friendly approach can be extended to other organic compounds of the aldehyde class that are important for medicine, agriculture, and the food industry. The article is published in the journal Molecular Catalysis.
Traditional methods of oxidation of alcohols to aldehydes and ketones using chromium and manganese lead to the formation of a large number of harmful byproducts that must be separately disposed of. To avoid this, one can use soft selective oxidizers—for example, hydrogen peroxide. However, this reaction requires catalysis; without it, the yield of the product is not more than 10 percent. But existing catalysts, such as silver phosphate, platinum and palladium compounds, are also toxic or expensive. Therefore, the task of finding a cheap, efficient and eco-friendly catalyst for industrial production of cinnamaldehyde remains relevant.
Chemists who create catalysts can solve several problems. Firstly, it is necessary to achieve high efficiency, that is, the catalyst should facilitate the reaction of the largest proportion of reagents incorporated in the initial mixture. Secondly, the catalyst must be highly selective—this means that the proportion of unwanted byproducts must be minimal. Thirdly, the catalyst should be safe for the environment. All these problems are relevant for the oxidation reactions of alcohols, which result in the formation of aldehydes and ketones—important compounds for organic synthesis, medicine, perfume industry or agriculture.
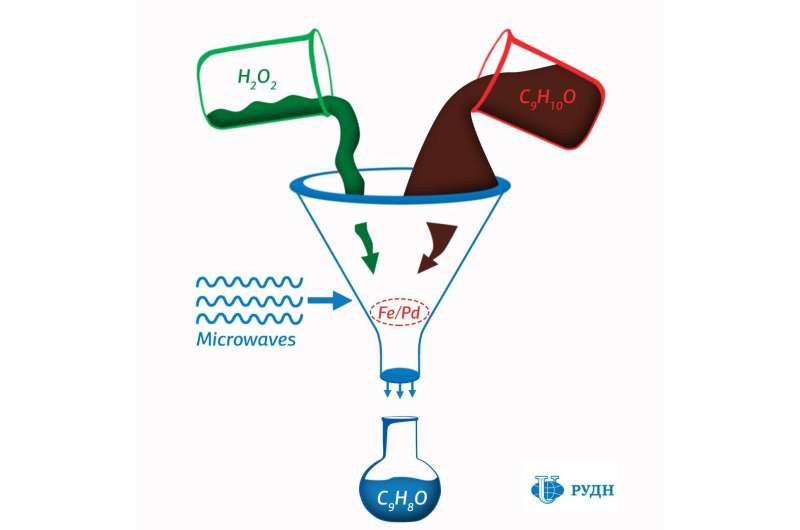
The research team led by RUDN University chemist Rafael Luque has proposed an effective and environmentally safe catalyst for the synthesis of cinnamaldehyde. This aromatic compound has antibacterial and anticarcinogenic activity and is widely used in the food and perfume industry as a flavoring agent, and in agriculture as a fungicide. One of the main ways to obtain it is the oxidation of cinnamyl alcohol.
Luque has proposed to use catalysts based on iron and palladium nanoparticles obtained by the mechanochemical method. These nanoparticles catalyze the selective oxidation of cinnamyl alcohol by hydrogen peroxide under microwave irradiation. At the same time, they are environmentally safe and relatively cheap.
However, in order for iron and palladium to work as catalysts, they must be applied to a suitable porous surface: its structure and chemical properties also determine the effectiveness of the entire catalytic system. As such matrices, chemists used several types of porous silicate and aluminosilicate substrates, which interact differently with oxygen and therefore lead to the formation of different products.
To assess the effectiveness of the catalyst, chemists measured the degree of conversion, that is, the proportion of alcohol converted to aldehyde. To test the selectivity of catalysts, scientists measured the ratio of cinnamaldehyde to an unwanted byproduct—benzaldehyde—in the final mixture. The most effective catalyst was a system of iron nanoparticles on an aluminosilicate zeolite matrix. The conversion rate for it was above 80 percent—higher than that of palladium catalysts and the solution of iron salts.
In this case, the catalyst of palladium nanoparticles on a magnetic silicate substrate surpassed the selectivity of all other options. After using this catalyst, the proportion of cinnamaldehyde in the final mixture exceeded 60 percent.
To understand why iron-based catalysts are more effective and palladium-based catalysts are more selective, the authors studied the underlying mechanism. They found that the oxidation of cinnamyl alcohol formed an intermediate product with an epoxy group,—because of it, more efficient catalysts accelerate simultaneously the oxidation reaction with the destruction of the carbon chain and the formation of shorter molecules of benzaldehyde, which reduces the selectivity.
Despite the fact that the high efficiency of the studied catalytic systems is associated with a decrease in selectivity, catalysts consist of iron nanoparticles on zeolite matrices have both indicators high enough for potential use in industrial production. The authors think that due to high activity of such catalysts—primarily based on iron nanoparticles—it can be used to oxidize not only cinnamyl alcohol but also other compounds with a similar chemical structure.
More information: Yantao Wang et al. Mechanistic insights into the microwave-assisted cinnamyl alcohol oxidation using supported iron and palladium catalysts, Molecular Catalysis (2019). DOI: 10.1016/j.mcat.2019.110409
Provided by RUDN University