Theoretical physicist predicted structure of gold cluster that chops carbon dioxide

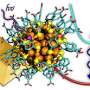
Gold and silver are important elements in cluster chemistry. They are chemically very active and are thus expected to be useful substances in future technologies. A doctoral dissertation from University of Jyvaskyla, Finland, shows successful computer simulations that were able to predict the atom-precise structure of a cluster comprising 11 gold atoms. Later, the compound was observed to speed up decomposition of carbon dioxide. The results can be used in developing catalysts.
Gold and silver nanoclusters can accelerate various chemical reactions. M.Sc. Sami Kaappa, a theoretical physicist, investigated nanometer-sized gold and silver clusters in his doctoral dissertation. "Speaking of nanoclusters, we usually mean relatively large molecules with a nucleus consisting of dozens of metal atoms, protected by monolayer of organic molecules so that the structure is stable in solution. Typically, the protecting layer consists of sulfur or phosphine ligands," Sami Kaappa says.
In his research, Kaappa used computer simulations to study properties of nanosized particles. The calculations were run on the Sisu supercomputer in Kajaani, Finland, and the Mare Nostrum supercomputer in Barcelona.
"Using simulations, we can predict possible chemical structures, even if there is only a little experimental data available," Kaappa says.
CO2-splitting cluster observed
One of the most remarkable findings of the dissertation research was the structure of a cluster with 11 gold atoms. The work was done in collaboration with experimental groups in Canada and Japan.
"The experimentalists were able to synthesize and realize the gold-11 cluster, but the atom-precise structure could not be determined at that point. Using computational methods, I could determine some possible structures, of which a single one seemed to be more favorable than others. Later, the experimental work verified this prediction," Kaappa says.
In the same study, they found that the decomposition of carbon dioxide is accelerated in the presence of the cluster.
"In the cluster, one of the phosphine ligands is exchanged to carbene, which is a new type of ligand in clusters. The kinds of observation of the catalytic activity may contribute to development of new technologies, such as catalytic converters or innovations in recycling harmful compounds. This is also fascinating for a theoretician since the reaction of carbon dioxide to carbon monoxide is still not known in detail," Sami Kaappa says.
More information: Sami Kaappa, Analysis and Applications of Electronic Structure in Gold and Silver Nanoclusters. JYU Dissertations, number 120, Jyväskylä, 2019. jyx.jyu.fi/handle/123456789/65304
Provided by University of Jyväskylä