Selective dissolution of elemental gold from multi-metal sources in organic solutions
"Urban mining", the recycling of precious metals from electronic gadgets, becomes ever more important, although processes that are both efficient and environmentally benign are still scarce. An international team of scientists has now looked deeper into gold dissolution, in particular, how organic thiol-containing compounds help dissolve elemental gold. Their study published in the journal Angewandte Chemie proposes selective, fast, and convenient thiol-assisted gold leaching processes.
The traditional way of recycling gold "waste" is melting: dental gold and jewelry can be recycled close to 100 percent. Recycling of precious metals in smartphones, computers, and other electronic gadgets is much harder, and the recovery quote is still low. Despite their abundance in electronic devices, their relative content is still too low to allow for really economical urban mining.
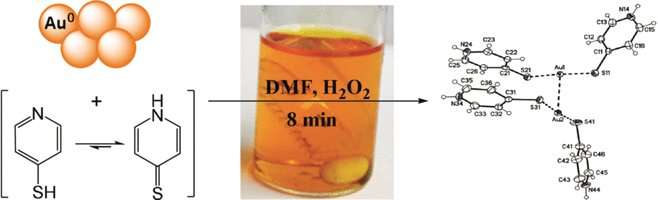
The traditional mining method for gold is hydrometallurgical cyanide leaching, which produces a vast amount of hazardous waste while being relatively unselective. More recent concepts rely on the complexation of gold in organic solutions because forms soluble complexes with sulfur-containing reagents. However, the processes must be feasible on a large scale and still avoid toxic or hazardous compounds. Now, Timo Repo at the University of Helsinki, Finland, and his colleagues have looked deeper into the details of selective gold extraction in organic solution. They propose an efficient gold recovery method from electronic waste with pyridinethiols and hydrogen peroxide as reagents, the chemical dimethyl formamide as organic solvent, and, optionally, elemental sulfur to reduce the reagent load.
Pyridinethiol is pyridine, a nitrogen-containing aromatic ring, with a thiol group, SH, added to its ring. The reagent not only binds elemental gold to form soluble complexes, but the complex has also a favorable linear structure formed by two pyridinethiol molecules on either side of the gold atom. Upon oxidation, it transforms to a stable cationic gold-containing product in organic solution. This complex formation with two ligands is a specialty of gold, favoring the energetics of dissolution and oxidation. Accordingly, the authors reported nearly quantitative dissolution of gold from powder, film, or electronic boards after 20 minutes extraction time.
But how can gold dissolution be distinguished from that of other precious metals? In contrast to gold having a one-electron oxidation, platinum and palladium require two-electron oxidations and thus are not accessible with this method. In contrast, both copper and silver form complexes with pyridinethiols, although not as effective as gold. Therefore, before dissolving the gold from the "gold finger" region in a printed circuit board, the scientists first extracted copper and silver with ammonia and sulfate-containing solutions, which are established methods.
Looking into the exact mechanism of thiol-assisted gold dissolution, the scientists discovered a surprisingly high variety of sulfur-containing side products. Some of them seemed to be crucial for proceeding the oxidation reaction, for example S8, a common form of elemental sulfur. This also proved to be an asset: By adding external S8, the ligand load could be reduced, reported the authors. Their extraction method could mark a new basis for more efficient urban mining.
More information: Minna Räisänen et al. Pyridinethiol-Assisted Dissolution of Elemental Gold in Organic Solutions, Angewandte Chemie International Edition (2018). DOI: 10.1002/anie.201810447
Journal information: Angewandte Chemie International Edition , Angewandte Chemie
Provided by Wiley