Study reveals how a small molecule promotes removal of excess cholesterol
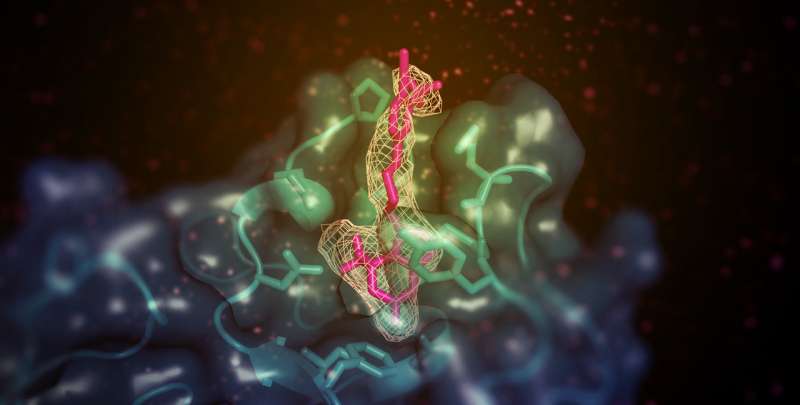
Scientists have determined the structure of the activated form of an enzyme that helps to return excess cholesterol to the liver, a study in eLife reports.
The research reveals how a drug-like chemical stimulates the action of the lecithin:cholesterol acyltransferase (LCAT) enzyme. It also suggests that future drugs using the same mechanism could be used to restore LCAT function in people with familial LCAT deficiency (FLD), a rare inherited disease that puts them at risk of eye problems, anaemia and kidney failure.
LCAT helps high-density lipoprotein (HDL) - known as the 'good' cholesterol—to remove cholesterol from the blood by converting the lipid into a form that is easier to package and transport. There are more than 90 known mutations in LCAT, which can cause either a partial loss of activity (known as 'fish-eye disease') or full loss (FLD). Boosting LCAT activity could therefore be beneficial in treating people with coronary heart disease and LCAT deficiencies, but the mechanisms by which it can be activated are poorly understood.
"In this study, we used structural biology to understand how a patented LCAT activator binds to LCAT and how it promotes cholesterol transport," says lead author Kelly Manthei, a Postdoctoral Fellow at the University of Michigan Life Sciences Institute, US. "We also asked if the compound could help recover activity of LCAT enzymes that have commonly observed mutations seen in FLD."
The team used X-ray crystallography to look at the LCAT enzyme stabilized in its active state with two different chemicals—the activator molecule, and a second compound that mimics a substrate bound to the enzyme. The two chemicals had more of an effect on the protein when presented together than when presented separately, which suggested that they bind to the enzyme in different places.
Further analysis found that the activator molecule, unlike other known LCAT activators, binds to a region close to where HDL attaches. However, the activator did not help LCAT bind to the HDL more effectively, which led the team to speculate that it instead helps to transfer cholesterol and lipids into the catalytic center of the enzyme, so that it can convert it into cargo for transport in HDL.
Having established this mode of action, the researchers tested whether this molecule could help recover the cholesterol-transport function of a mutant LCAT enzyme. They made a version of the enzyme with a mutation commonly seen in FLD patients, and then tested its ability to bind to HDL and convert cholesterol in the presence or absence of the activator molecule. They were excited to find that the activator could partly reverse the loss of activity in the mutant enzymes, resulting in comparable cholesterol conversion to the normal enzyme.
"Our results will help scientists design compounds that can better target LCAT so they might be of therapeutic benefit for heart disease and FLD patients," concludes senior author John Tesmer, Walther Professor in Cancer Structural Biology at Purdue University, US. "Future efforts will be to examine whether patients with other LCAT genetic mutations could benefit from the compounds used in this study, and to design molecules with improved pharmacological properties for further development."
More information: Kelly A Manthei et al, Molecular basis for activation of lecithin:cholesterol acyltransferase by a compound that increases HDL cholesterol, eLife (2018). DOI: 10.7554/eLife.41604
Journal information: eLife
Provided by eLife