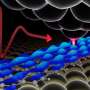
How do peptides penetrate cells? Two sides of the same coin
The simple transport of drugs directly into cells is one of the primary goals of the pharmaceutical industry. In large part, researchers still don't possess a detailed understanding at the molecular level of the processes responsible for transporting substances into and out of cells. In collaboration with colleagues from the Czech Republic and Germany, the research team of Pavel Jungwirth from the Institute of Organic Chemistry and Biochemistry of the Czech Academy of Sciences (IOCB Prague) has discovered a previously unknown mechanism by which short peptides are able to penetrate cells and in principle, could serve as carriers of drug molecules. The results of their research have now been published in the Proceedings of the National Academy of Sciences .
The ability of positively charged short peptides to penetrate cells was first observed in HIV research, and today, it's gradually being employed to transport drugs into cells. Until now, however, this primarily took the form of so-called vesicular transport, i.e., by means of a transport vesicle separating from the cell membrane and enveloping the transported substance, which must then break free from the vesicle after transport into the cell is complete, potentially posing a technical complication for efficient transportation of the drug. It's known that peptides can also penetrate cells passively, i.e. independently of energy from the cell, but the exact mechanism has yet to be described.
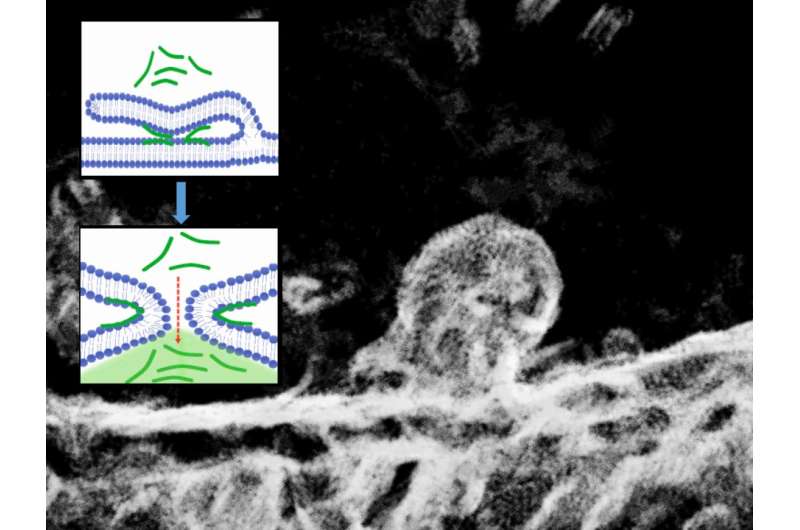
Now, using fluorescence and electron microscopy in combination with molecular computer simulations, the scientists have discovered a previously undescribed passive mechanism for transporting positively charged peptides into cells. It's based on membrane fusion induced by the transported peptides (see the image). The scientists have thus demonstrated that the process of passive transport of peptides into cells and the widely known process of membrane fusion—for instance, that induced by calcium ions in neurons during the transmission of nerve impulses—share the same mechanistic basis. Figuratively speaking, they're two sides of the same coin.
"At this point, we can only speculate as to practical applications for the discovery," says Jungwirth. "If, however, this newly discovered mechanism proves sufficiently robust, in the future, we could consider the possibility of passively transporting drug molecules into cells without having to free them from vesicles, which, in this process, simply don't form."
Jungwirth and his team have long focused on revealing the laws governing molecular processes in the cell membrane, which, to this day, are still largely unknown. A better understanding of the basic processes occurring within the membranes of cells gradually opens the door to new possibilities for controlling these processes and thereby conceivably more efficient methods of transporting drug molecules to their site of action.
More information: Christoph Allolio et al, Arginine-rich cell-penetrating peptides induce membrane multilamellarity and subsequently enter via formation of a fusion pore, Proceedings of the National Academy of Sciences (2018). DOI: 10.1073/pnas.1811520115
Journal information: Proceedings of the National Academy of Sciences
Provided by Institute of Organic Chemistry and Biochemistry of the Czech Academy of Sciences (IOCB Prague)