New method enables large-scale production of bio-based plastic bottles
Scientists have discovered a novel method to synthesize furan-2,5-dicarboxylic acid (FDCA) in a high yield from a glucose derivative of non-food plant cellulose, paving the way for replacing petroleum-derived terephthalic acid with biomaterials in plastic bottle applications.
The chemical industry is under pressure to establish energy-efficient chemical procedures that do not generate by-products, and using renewable resources wherever possible. Scientists believe that if resources from non-food plants can be used without putting a burden on the environment, it will help sustain existing social systems.
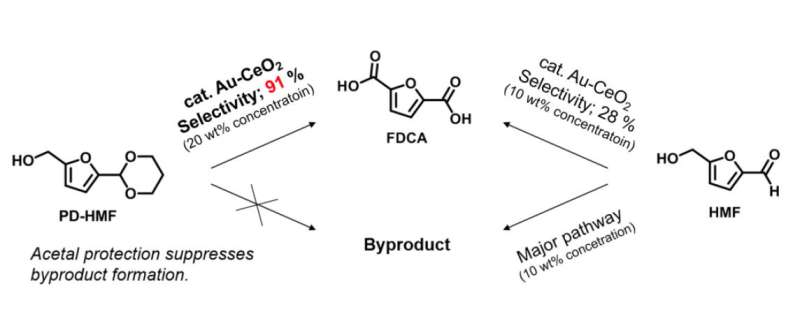
It has been reported that various useful polymers can be synthesized from 5-(hydroxymethyl)furfural (HMF), the biomaterial used in this study. A high yield of FDCA can be obtained when HMF is oxidized in a diluted solution under 2 weight percentage (wt percent) with various supported metal catalysts. However, a major stumbling block to industrial application lies with the use of a concentrated solution of 10-20 wt percent, which is essential for efficient and scalable production of FDCA in the chemical industry. When HMF was simply oxidized in a concentrated solution (10 wt percent), the FDCA yield was only around 30 percent, and a large amount of solid by-products was formed simultaneously. This is due to complex side reactions induced from HMF itself.
In the study published in Angewandte Chemie International Edition, a Japan-Netherlands research team led by Associate Professor Kiyotaka Nakajima at Hokkaido University and Professor Emiel J.M. Hensen at Eindhoven University of Technology succeeded in suppressing the side reactions and producing FDCA with high yields from concentrated HMF solutions (10~20 wt percent) without by-products formation. Specifically, they first acetalized HMF with 1,3-propanediol to protect by-product-inducing formyl groups and then oxidized HMF-acetal with a supported Au catalyst.
About 80 percent of 1,3-propanediol used to protect formyl groups can be reused for the subsequent reactions. In addition, drastic improvement in the substrate concentration reduces the amount of solvents used in the production process. Kiyotaka Nakajima says "It is significant that our method can reduce the total energy consumption required for complex work-up processes to isolate the reaction product."
"These results represent a significant advance over the current state of the art, overcoming an inherent limitation of the oxidation of HMF to an important monomer for biopolymer production. Controlling the reactivity of formyl group could open the door for the production of commodity chemicals from sugar-based biomaterials," says Kiyotaka Nakajima. This study was conducted jointly with Mitsubishi Chemical Corporation.
Scientists have discovered a novel method to synthesize furan-2,5-dicarboxylic acid (FDCA) in a high yield from a glucose derivative of non-food plant cellulose, paving the way for replacing petroleum-derived terephthalic acid with biomaterials in plastic bottle applications.
The chemical industry is under pressure to establish energy-efficient chemical procedures that do not generate by-products, and using renewable resources wherever possible. Scientists believe that if resources from non-food plants can be used without putting a burden on the environment, it will help sustain existing social systems.
It has been reported that various useful polymers can be synthesized from 5-(hydroxymethyl)furfural (HMF), the biomaterial used in this study. A high yield of FDCA can be obtained when HMF is oxidized in a diluted solution under 2 weight percentage (wt percent) with various supported metal catalysts. However, a major stumbling block to industrial application lies with the use of a concentrated solution of 10-20 wt percent, which is essential for efficient and scalable production of FDCA in the chemical industry. When HMF was simply oxidized in a concentrated solution (10 wt percent), the FDCA yield was only around 30 percent, and a large amount of solid by-products was formed simultaneously. This is due to complex side reactions induced from HMF itself.
In the study published in Angewandte Chemie International Edition, a Japan-Netherland research team led by Associate Professor Kiyotaka Nakajima at Hokkaido University and Professor Emiel J.M. Hensen at Eindhove University of Technology succeeded in suppressing the side reactions and producing FDCA with high yields from concentrated HMF solutions (10~20 wt percent) without by-products formation. Specifically, they first acetalized HMF with 1,3-propanediol to protect by-product-inducing formyl groups and then oxidized HMF-acetal with a supported Au catalyst.
About 80 percent of 1,3-propanediol used to protect formyl groups can be reused for the subsequent reactions. In addition, drastic improvement in the substrate concentration reduces the amount of solvents used in the production process. Kiyotaka Nakajima says "It is significant that our method can reduce the total energy consumption required for complex work-up processes to isolate the reaction product."
"These results represent a significant advance over the current state of the art, overcoming an inherent limitation of the oxidation of HMF to an important monomer for biopolymer production. Controlling the reactivity of formyl group could open the door for the production of commodity chemicals from sugar-based biomaterials," says Kiyotaka Nakajima. This study was conducted jointly with Mitsubishi Chemical Corporation.
More information: Minjune Kim et al. Aerobic Oxidation of 5-(Hydroxymethyl)furfural Cyclic Acetal Enables Selective Furan-2,5-dicarboxylic Acid Formation with CeO2 -Supported Gold Catalyst, Angewandte Chemie International Edition (2018). DOI: 10.1002/anie.201805457
Journal information: Angewandte Chemie International Edition
Provided by Hokkaido University




















