Atoms use tunnels to escape graphene cover
Graphene has held great potential for practical applications since it was first isolated in 2004. But we still don't use it in our large-scale technology, because we have no way of producing graphene on an industrial scale. Physicists from Leiden University have now visualized for the first time how atoms behave in between graphene and a substrate. This insight could be instrumental for future implementations of industrial graphene production. Their results have been published in Physical Review Materials.
In 2004, scientists isolated a single layer of carbon atoms from a block of graphite. Graphene layers could enable high-speed transistors, inexpensive electrical cars and delicate sensors. Fast-forward to 2018, and graphene there are still few large-scale graphene applications. The problem is that researchers haven't figured out a way to produce graphene in high quality on the right substrate on an industrial scale.
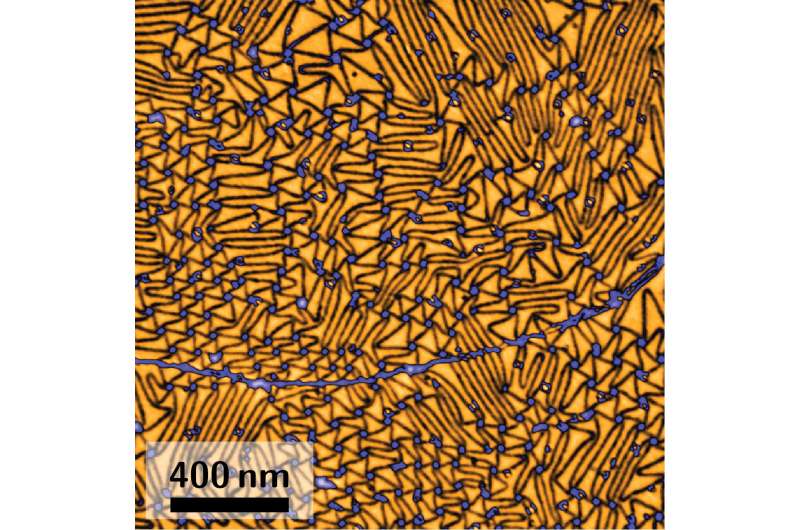
Though scientists do have an idea for large-scale production: Heat silicon carbide to almost 2,000 degrees C, and a graphene layer grows on its surface. However, researchers need to make sure that the desired properties of the graphene are not disturbed by the substrate. Inserting hydrogen atoms in between the graphene and silicon carbide isolates the graphene and leaves it intact as a single-layer material. Physicist Sense Jan van der Molen and his research group at Leiden University have now visualized for the first time how those atoms behave underneath the graphene.
The researchers, including postdoc Johannes Jobst and PhD candidate Tobias de Jong, used their low-energy electron microscope (LEEM) to study what happens to hydrogen atoms sandwiched between graphene and silicon carbide. They spotted lines where the graphene layer is strained. The hydrogen atoms use the lines as tunnels where they can escape more easily, whereas they stay put much longer under the graphene's smooth regions between these lines. "The reversed process is widely used in research to decouple the graphene from the substrate," says Jobst. "But it was not clear how the hydrogen moves at the interface. We could show that hydrogen gas can be blown into those tunnels so that it will spread quickly underneath the graphene layer in the form of individual atoms."
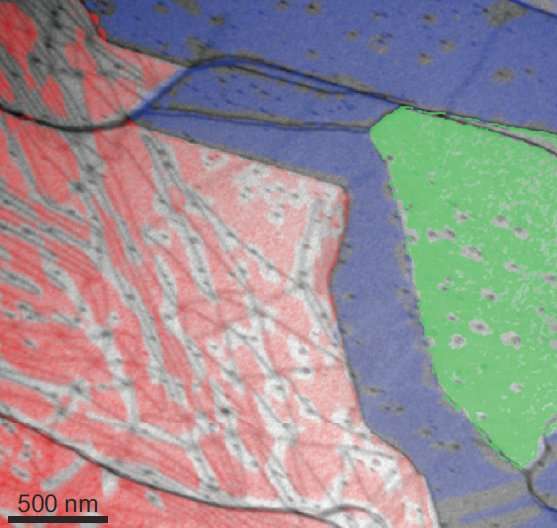
More information: T. A. de Jong et al. Intrinsic stacking domains in graphene on silicon carbide: A pathway for intercalation, Physical Review Materials (2018). DOI: 10.1103/PhysRevMaterials.2.104005
Provided by Leiden University