New chemical method could revolutionize graphene
University of Illinois at Chicago scientists have discovered a new chemical method that enables graphene to be incorporated into a wide range of applications while maintaining its ultra-fast electronics.
Graphene, a lightweight, thin, flexible material, can be used to enhance the strength and speed of computer display screens, electric/photonics circuits, solar cells and various medical, chemical and industrial processes, among other things. It is comprised of a single layer of carbon atoms bonded together in a repeating pattern of hexagons.
Isolated for the first time 15 years ago by a physics professor at the University of Manchester in England, it is so thin that it is considered two-dimensional and thought to be the strongest material on the planet.
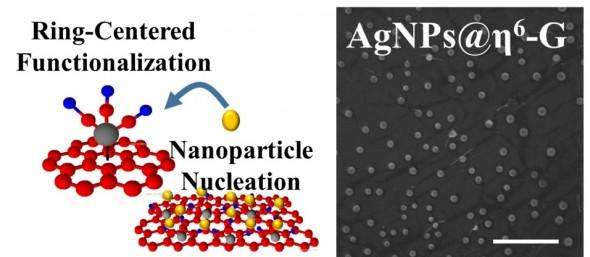
Vikas Berry, associate professor and department head of chemical engineering, and colleagues used a chemical process to attach nanomaterials on graphene without changing the properties and the arrangement of the carbon atoms in graphene. By doing so, the UIC scientists retained graphene's electron-mobility, which is essential in high-speed electronics.
The addition of the plasmonic silver nanoparticles to graphene also increased the material's ability to boost the efficiency of graphene-based solar cells by 11 fold, Berry said.
The research, funded by the National Science Foundation (CMMI-1030963), has been published in the journal Nano Letters.
Instead of adding molecules to the individual carbon atoms of graphene, Berry's new method adds metal atoms, such as chromium or molybdenum, to the six atoms of a benzoid ring. Unlike carbon-centered bonds, this bond is delocalized, which keeps the carbon atoms' arrangement undistorted and planar, so that the graphene retains its unique properties of electrical conduction.
The new chemical method of annexing nanomaterials on graphene will revolutionize graphene technology by expanding the scope of its applications, Berry said.
"It's been a challenge to interface graphene with other nano-systems because graphene lacks an anchoring chemistry," he said. "And if graphene's chemistry is changed to add anchors, it loses its superior properties. The distinction of our chemistry will enable integration of graphene with almost anything, while retaining its properties.
"We envision that our work will motivate a worldwide move towards 'ring-centered' chemistries to interface graphene with other systems."
More information: Songwei Che et al. Retained Carrier-Mobility and Enhanced Plasmonic-Photovoltaics of Graphene via ring-centered ?Functionalization and Nanointerfacing, Nano Letters (2017). DOI: 10.1021/acs.nanolett.7b01458
Journal information: Nano Letters
Provided by University of Illinois at Chicago