Researchers report new oxyfluoride compound for photocatalysis
Over the last decade, research has intensified to develop efficient, manmade photocatalysts that work under visible light—an important target for renewable energy systems. Scientists in Japan have now shown that an oxyfluoride is capable of visible light-driven photocatalysis. The finding opens new doors for designing materials for artificial photosynthesis and solar energy research.
Kazuhiko Maeda of Tokyo Institute of Technology (Tokyo Tech), Kengo Oka of Chuo University and collaborators in Japan have succeeded in demonstrating that Pb2Ti2O5.4F1.2 works as a stable photocatalyst for visible light-driven water splitting and carbon dioxide reduction with the aid of proper surface modifications.
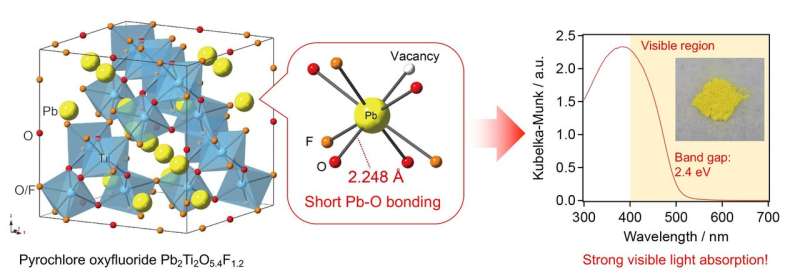
The new material has an unusually small band gap of around 2.4 electron volts (eV), meaning that it can absorb visible light with a wavelength of around 500 nanometers (nm). In general, band gaps bigger than 3 eV are associated with inefficient utilization of sunlight, whereas those smaller than 3 eV are desirable for efficient solar energy conversion.
Additionally, the oxyfluoride belongs to a group of compounds that had until now been largely overlooked due to the highest electronegativity of fluorine, a property that essentially ruled them out as candidates for visible light-driven photocatalysts.
The new oxyfluoride is "an exceptional case," the researchers say in their study, which is published in the Journal of the American Chemical Society.
Based on structural considerations and theoretical calculations, they conclude that "the origin of the visible light response in Pb2Ti2O5.4F1.2 lies in the unique features specific to the pyrochlore-type structure." Namely, it is the strong interaction between certain orbitals (Pb-6s and O-2p) enabled by short Pb-O bonding in the pyrochlore structure that is thought to give rise to the material's ability to absorb visible light.
One limitation is that the yield of the new photocatalyst currently remains low, at a figure of around 0.01 percent at 365 nm for hydrogen evolution. The research team is therefore investigating how to boost the yield by modifying Pb2Ti2O5.4F1.2 through refinement of methods for synthesis and surface modification.
More information: Ryo Kuriki et al, A Stable, Narrow-Gap Oxyfluoride Photocatalyst for Visible-Light Hydrogen Evolution and Carbon Dioxide Reduction, Journal of the American Chemical Society (2018). DOI: 10.1021/jacs.8b02822
Journal information: Journal of the American Chemical Society
Provided by Tokyo Institute of Technology



















