Cryo-EM reveals 'crown-like' structure of protein responsible for regulating blood flow
A team led by scientists at Van Andel Research Institute (VARI) has revealed for the first time the atomic-level structure of a promising drug target for conditions such as stroke and traumatic brain injury.
Called TRPM4, this protein is found in tissues throughout the body, including the brain, heart, kidney, colon and intestines, where it plays a major role in regulating blood flow via blood vessel constriction as well as setting the heart's rhythm and moderating immune responses.
"Understanding the role TRPM4 plays in regulating circulation is vital, but for years research has been limited by a lack of insight about its molecular architecture," said Wei Lü, Ph.D., an assistant professor at VARI and lead author on a study describing TRPM4's structure, published today in Nature. "Our findings not only provide a detailed, atomic-level map of this critical protein, but also reveal completely unexpected facets of its makeup."
TRPM4 is critically involved in regulating the blood supply to the brain, which comprises only about 2 percent of the body's total weight yet receives 15 to 20 percent of its blood supply. Conditions that disrupt blood flow in the brain, such as stroke, traumatic brain injury, cerebral edema and hypertension, can have devastating consequences and are significant public health problems.
"Many safeguards exist in the brain's circulatory system to protect against a sudden interruption in blood supply, one of which is TRPM4," Lü said. "We hope that a better understanding of what this protein looks like will give scientists a molecular blueprint on which to base the design of more effective medications with fewer side effects."
The structure of TRPM4 is markedly different from the other molecules in the TRP superfamily, a category of proteins that mediate responses to sensations and sensory stimuli, such as pain, pressure, vision, temperature and taste. Broadly known as ion channels, proteins like TRP nestle within cells' membranes, acting as gatekeepers for chemical signals passing into and out of the cell.
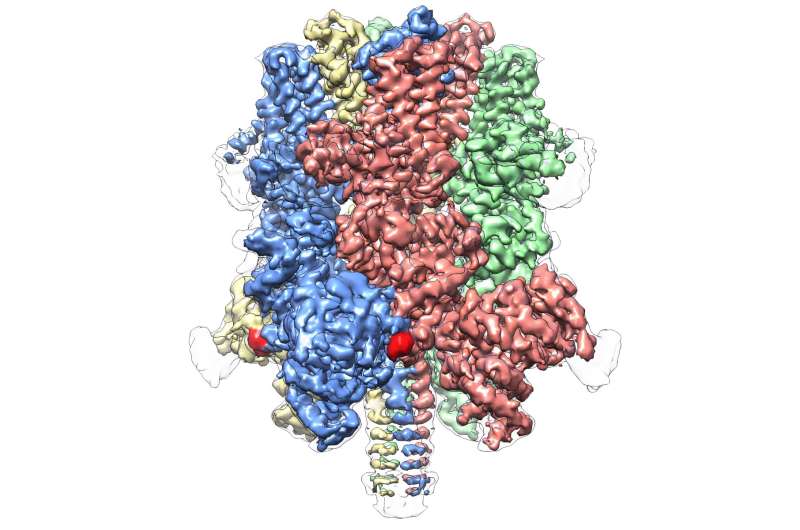
Even within its own subfamily, which comprises eight molecules in total, TRPM4 appears to be wholly unique. Today's publication represents the first atomic view of a member of the TRPM subfamily.
It reveals a crown-like structure, with the four peaks composing a large N-terminal domain—a hallmark of TRPM proteins. This region, found at the start of the molecule, is a major site of interaction with the cellular environment and other molecules in the body. On the opposite end of TRPM4, commonly called the C-terminal domain, Lü's team found an umbrella-like structure supported by a "pole" and four helical "ribs"—characteristics that have never before been observed.
The findings were made possible by VARI's state-of-the-art David Van Andel Advanced Cryo-Electron Microscopy Suite, which allows scientists to view some of life's smallest components in exquisite detail. VARI's largest microscope, the Titan Krios, is one of fewer than 120 in the world and is so powerful that it can visualize molecules 1/10,000th the width of a human hair.
Lü's structure is the second molecular structure determined on the Institute's Krios since completion of the suite's installation earlier this year.
More information: Paige A. Winkler et al. Electron cryo-microscopy structure of a human TRPM4 channel, Nature (2017). DOI: 10.1038/nature24674
Journal information: Nature
Provided by Van Andel Research Institute