Sharpest image of Alzheimer's fibrils shows previously unknown details
A team of researchers from Germany and the Netherlands has determined the structure of an amyloid fibril with previously unachieved resolution. The fibrils of the body's own amyloid beta (Aβ) protein are the main constituent of brain protein deposits associated with Alzheimer's. The atomic-level three-dimensional structure elucidated by the scientists reveals previously unknown aspects of the growth of harmful deposits and the effect of genetic risk factors. The results have been published in the renowned journal Science.
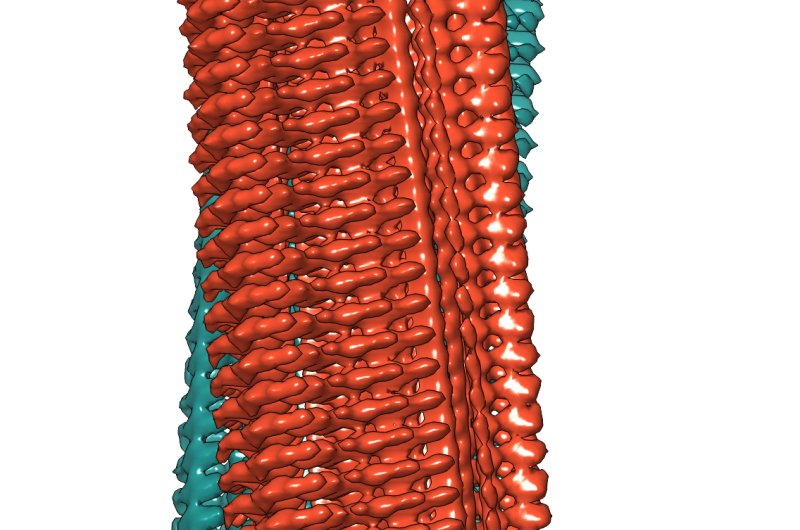
The structure reveals how the many single Aβ protein molecules are staggered in layers on top of each other and are arranged into so-called protofilaments. Two of these protofilaments are twinned around each other to form a fibril. If several of these fibrils become entangled, they give rise to the characteristic deposits or plaques that are detected in the brain tissues of Alzheimer's patients.
"This is a milestone on the road to a fundamental understanding of amyloid structures and related diseases," says Prof. Dieter Willbold. "The fibril structure answers many questions about the mechanism of fibril growth and identifies the role played by a whole series of familial mutations that lead to early onset of Alzheimer's disease."
The resolution of 4 angstroms, corresponding to 0.4 nanometres, is within the typical magnitude of atomic radii and atomic bond lengths. In contrast to previous work, the model shows for the first time the exact position and interactions of the proteins. The Aβ molecules of the entangled protofilaments are thus not at the same level, but like a zipper, they are staggered by half an interval. Furthermore, the structure elucidates the location and conformation of all 42 amino acid residues of the many individual Aβ protein molecules for the first time.
This detailed structure provides a new basis for understanding the structural effect of a number of genetic modifications that increase the risk of developing the disease. They stabilize the fibrils by changing the blueprint of the protein at defined locations. This also explains why in nature mice do not develop Alzheimer's, and why a small section of the Icelandic population seems to be more or less resistant to the disease. Their variants of Aβ differ by three or one amino acid residues, respectively, which are apparently important for the stability of the fibrils.
Methodological diversity at the highest technological level
In contrast to the plaques which are typical for the disease discovered by Alois Alzheimer more than 100 years ago, the fibril structure now uncovered cannot be directly observed under the light microscope. It took more than a year to analyse the data the scientists had obtained using the cryo-electron microscopy facility at Maastricht University. Moreover, measurements using solid-state nuclear magnetic resonance (NMR) spectroscopy and X-ray diffraction experiments helped to supplement and fully support the picture of the fibril structure and validate the data obtained.
"The individual images in cryo-electron microscopy are usually extremely noisy since proteins are very sensitive to electron radiation and the pictures can only be generated with very low radiation intensity," explains Jun.-Prof. Gunnar Schröder. Using a computer-assisted procedure, he combined thousands of individual images and thus extracted high-resolution structural data from them.
"This is a step that can be very complicated if the sample consists of differently formed fibrils. In the past, this was almost always the case with the amyloid fibrils and represented one of the major obstacles for the analysis. However, we now had a fairly unique specimen with very homogeneous fibrils—90 percent of them had the same shape and symmetry," says Schröder.
Dr. Lothar Gremer succeeded in producing the fibril specimen. "The crucial step was to greatly retard the growth of the fibrils in the specimen, from a few hours to several weeks. Thereby, the individual Aβ molecules got enough time to arrange themselves into homogeneous fibrils in a very uniform and highly ordered way," says Gremer, who initiated and coordinated the study.
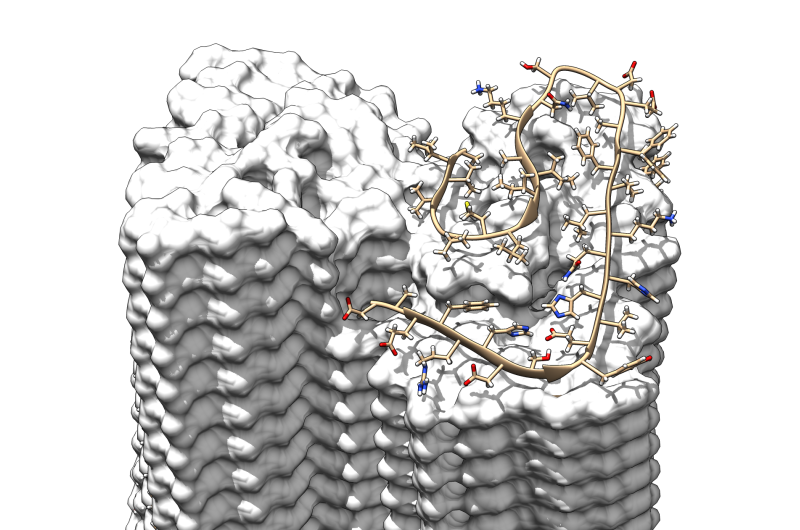
Investigations of the fibril specimen by solid-state nuclear magnetic resonance spectroscopy provided additional data to build the model and helped to validate the structure. "NMR enabled us to obtain additional information such as which amino acid residues form salt bridges thus enhancing the stability of the fibrils," explains Prof. Henrike Heise. X-ray diffraction experiments supervised by Prof. Jörg Labahn at the Centre for Structural Systems Biology in Hamburg additionally confirmed the results.
Cryo-electron microscopy is a relatively new research method for determining the structure of protein molecules. In the past, scientists mainly used X-ray crystallography and nuclear magnetic resonance spectroscopy. In 2015, cryo-electron microscopy was elected as research method of the year by the journal Nature Methods. With the long-established method of X-ray crystallography, the proteins first have to be converted into a crystalline form, whereas with cryo-electron microscopy and also NMR spectroscopy, the protein building blocks can be investigated in their natural state. In the case of cryo-electron microscopy, the specimens are first dissolved in water, then flash frozen, and finally investigated with an electron microscope. This method has particular advantages when it comes to investigating large structures composed of hundreds or thousands of proteins.
The establishment of a facility for high-resolution cryo-electron microscopy could give scientists at Jülich the opportunity to investigate biological molecules. In addition to basic research, Jülich's Institute of Complex Systems (ICS-6) is also developing a novel treatment strategy with its own drug candidate.
More information: "Fibril structure of amyloid-β(1-42) by cryoelectron microscopy" Science (2017). science.sciencemag.org/lookup/ … 1126/science.aao2825
Journal information: Science , Nature Methods
Provided by Forschungszentrum Juelich