Physicists discover a tri-anion particle with colossal stability
Virginia Commonwealth University researchers have achieved a feat that is a first in the fields of physics and chemistry—one that could have wide-ranging applications.
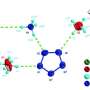
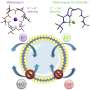
A team in the lab of Puru Jena, Ph.D., a distinguished professor in the Department of Physics in the College of Humanities and Sciences, has created the most stable tri-anion particle currently known to science. A tri-anion particle is a combination of atoms that contains three more electrons than protons. This discovery is novel because previously known tri-anion particles were unstable due to their numerical imbalance. These unstable particles dispel additional electrons, interrupting chemical reactions.
Jena partnered with Tianshan Zhao, a graduate student in the physics department; Jian Zhou, Ph.D., a postdoctoral fellow; and Qian Wang, Ph.D., a physics professor at Peking University, to use quantum mechanical calculations to create computer models to prove the stability of the BeB11(CN)12 tri-anion. This tri-anion is made of the elements boron and beryllium and the chemical compound cyanogen.
The researchers' work will be featured on the cover of Angewandte Chemie, a world-renowned chemistry journal, on Oct. 17. The team's article was designated a VIP paper by the publication, which means it is considered among the top five percent of papers for its contribution to the study of chemistry.
"This is very important in this field, nobody has ever found such a tri-anion," Jena said. "Not only can it keep three electrons but the third electron is extremely stable. The guiding principles we have used in this paper will help with the design of other tri-anions. The question is: What do we do with this knowledge?"
Real world applications
The tri-anion may have a number of industrial applications. So far, Jena and his team have hypothesized that the particle may be used in the creation of an aluminum ion battery, which has distinct advantages over the widely used rechargeable lithium ion battery. Aluminum is in greater supply than lithium and is less reactive. During the chemical reaction that would power the battery, the tri-anion would make the battery conductive by moving from one of its electrodes to the other.
While a battery is the only demonstrated use so far, existing applications for other particles with one additional electron, called mono-anions, and two additional electrons, called di-anions, show the potential of Jena's work.
"Such particles are very important for many reasons. Number one, they make salts. Secondly, they are used in all kinds of chemical compounds, such as those in floor cleaners as oxidizing agents that kill bacteria," Jena said. "They are also used to purify air, which is a billion-dollar industry, and in mood enhancers, similar to what Prozac does. The potential uses are endless."
More information: Tianshan Zhao et al, Colossal Stability of Gas-Phase Trianions: Super-Pnictogens, Angewandte Chemie International Edition (2017). DOI: 10.1002/anie.201708386
Journal information: Angewandte Chemie , Angewandte Chemie International Edition
Provided by Virginia Commonwealth University