February 15, 2017 report
Researchers characterize pentazolate anion as part of a stable salt
(Phys.org)—The pentazole molecule and its anion, cyclo-N5- has proven elusive to researchers for almost a century. The pentazole anion is highly unstable and cannot be made in bulk. Researchers from Nanjing University of Science and Technology and the University of Science and Technology Liaoning have devised a synthesis of a salt containing the pentazole anion that is stable up to 117oC. Their report appears in Science.
The pentazole and its anion have a sordid history, at least for molecules. Pentazole was thought to be isolated as an arylpentazole in 1915, but was disproven several years later. Then, in the 1950s, a group of researchers managed to identify pentazole as an intermediate for another reaction. Later researchers became interested in combining N5+ and N5- as a possible alternative to hydrazine as rocket fuel.
Many attempts have been made to isolate the pentazole anion by cleaving the carbon-nitrogen bond in an arylpentazole. However, these have proven unsuccessful because of the difficulties in selectively cleaving the carbon-nitrogen bond. The addition of electron donating groups at the ortho and para positions of the arene helped with selective cleavage.
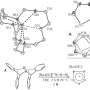
In the current study, Zhang et al. were able to selectively cleave the carbon-nitrogen bond of 3,5-dimethyl-4-hydroxyphenylpentazole and stabilize the resulting pentazole anion using ferrous bisglycinate, Fe(Gly)2. The anion was isolated as part of a salt, (N5)6(H3O)3(NH4)4Cl (19% yield). The Fe(Gly)2 stabilizer also served as a mediator for m-chloroperbenzoic acid.
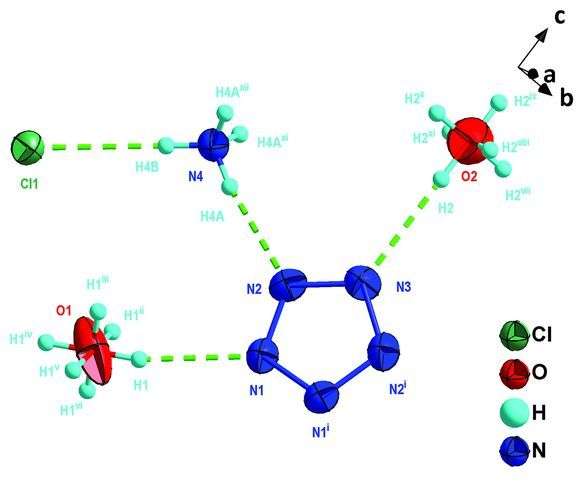
The molecular conformation of the salt ions was determined using single-crystal x-ray diffraction analysis where the five nitrogen atoms in cylco-N5- are co-planar and aromatic. The structure was confirmed using 1H and 15N NMR as well as infrared and Raman spectroscopy.
Additional studies showed that the salt is remarkably thermally stable up to 117oC, which is attributed to the salt's hydrogen bonding arrangement. All of the ions in the salt apparently play a role in stabilizing the pentazole anion. When Zhang et al. removed Cl- or when they removed NH4+, cyclo-N5- decomposed.
This research allows for the isolation and characterization of an elusive aromatic azole molecule, one that has been out of reach for chemists for many years, and according to the authors this ends the search for this elusive molecule.
More information: Chong Zhang et al. Synthesis and characterization of the pentazolate anion-Nˉ in (N)(HO)(NH)Cl, Science (2017). DOI: 10.1126/science.aah3840
Abstract
Pentazole (HN5), an unstable molecular ring comprising five nitrogen atoms, has been of great interest to researchers for the better part of a century. We report the synthesis and characterization of the pentazolate anion stabilized in a (N5)6(H3O)3(NH4)4Cl salt. The anion was generated by direct cleavage of the C–N bond in a multisubstituted arylpentazole using m-chloroperbenzoic acid and ferrous bisglycinate. The structure was confirmed by single-crystal x-ray diffraction analysis, which highlighted stabilization of the cyclo-N5ˉ ring by chloride, ammonium, and hydronium. Thermal analysis indicated the stability of the salt below 117°C on the basis of thermogravimetry-measured onset decomposition temperature.
Journal information: Science
© 2017 Phys.org