Similarities in human and pig embryos provide clues to early stages of development
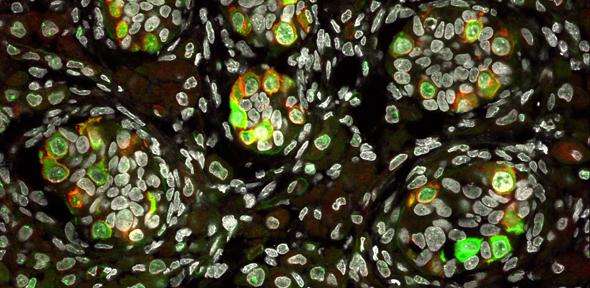
Scientists have shown how the precursors of egg and sperm cells – the cells that are key to the preservation of a species – arise in the early embryo by studying pig embryos alongside human stem cells.
In research published today in Nature, researchers at the University of Cambridge and the University of Nottingham demonstrate how pig embryos and human embryonic cells show remarkable similarities in the early stages of their development. By combining these two models, they hope to improve our understanding of the origins of diseases such as paediatric germ cell tumours and fetal abnormalities.
Primordial germ cells, the precursors of eggs and sperm, are among the earliest cells to emerge in human embryos after implantation, appearing around day 17, while the surrounding cells go on to form the rest of the human body. However, little is understood about how they originate. Currently, the law prohibits culture of human embryos beyond 14 days, which prevents investigations on this and subsequent events such as gastrulation, when the overall body plan is established.
Now, researchers have used a combination of human and pig models of development to shed light on these events. They have shown for the first time that the interplay between two key genes is critical for the formation of the germline precursors and that this 'genetic cocktail' is not the same in all species.
First, by using human pluripotent embryonic stem cells in vitro, scientists led by Professor Azim Surani at the Wellcome Trust/Cancer Research UK Gurdon Institute established a model that simulates genetic and cellular changes occurring up to gastrulation. Human pluripotent embryonic stem cells are 'master cells' found in embryos, which have the potential to become almost any type of cell in the body.
As these stem cells can be multiplied and precisely genetically manipulated, the model system provides a powerful tool for detailed molecular analysis of how human cells transform into distinct cell types during early development, and which changes might underlie human diseases.
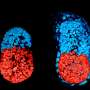
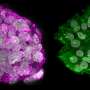
The work shows that when an embryo progresses towards gastrulation, cells temporarily acquire the potential to form primordial germ cells, but shortly afterwards lose this potential and instead acquire the potential to form precursors of blood and muscle (mesoderm) or precursors of the gut, lung and the pancreas (endoderm). The model also tells us that while the genes SOX17 and BLIMP1 are critical for germ cell fate, SOX17 subsequently has another role in the specification of endodermal tissues.
For an accurate picture of how the embryo develops, however, it is necessary to understand how cells behave in the three-dimensional context of a normal embryo. This cannot be achieved by studies on the most commonly used mouse embryos, which develop as egg 'cylinders', unlike the 'flat-disc' human embryos. Pig embryos, on the other hand, develop as flat discs (similar to human embryos), can be easily obtained, and are ethically more acceptable than working with non-human primate (monkey) embryos.
Researchers from the University of Nottingham dissected whole flat discs from pig embryos at different developmental stages and found that development of these embryos matches with the observations on the in vitro human model, as well as with non-human primate embryonic stem cells in vitro. For example, pig germ cells emerge in the course of gastrulation just as predicted from the human model, and with the expression of the same key genes as in human germ cells. Human and pig germ cells also exhibit key characteristics of this lineage, including initiation of reprogramming and re-setting of the epigenome – modifications to our DNA that regulate its operations and have the potential to be passed down to our offspring – which continues as germ cells progress towards development into sperm and eggs.
The combined human-pig models for early development and cell fate decisions likely reflect critical events in early human embryos in the womb. Altogether, knowledge gained from this approach can be applied to regenerative medicine for the derivation of relevant human cell types that might be used to help understand and treat human diseases, and to understand how mutations that perturb early development can result in human diseases.
Dr Ramiro Alberio, from the School of Biosciences at the University of Nottingham, says: "We've shown how precursors to egg and sperm cells arise in pigs and humans, which have similar patterns of embryo development. This suggests that the pig can be an excellent model system for the study of early human development, as well as improving our understanding of the origins of genetic diseases."
Dr Toshihiro Kobayashi in the Surani lab at the Gurdon Institute, adds: "We are currently prevented from studying human embryo development beyond day 14, which means that certain key stages in our development remain a mystery. The remarkable similarities between human and pig development suggest that we may soon be able to reveal the answers to some of our long-held questions."
More information: Toshihiro Kobayashi et al. Principles of early human development and germ cell program from conserved model systems, Nature (2017). DOI: 10.1038/nature22812
Journal information: Nature
Provided by University of Cambridge