New device produces hydrogen peroxide for water purification
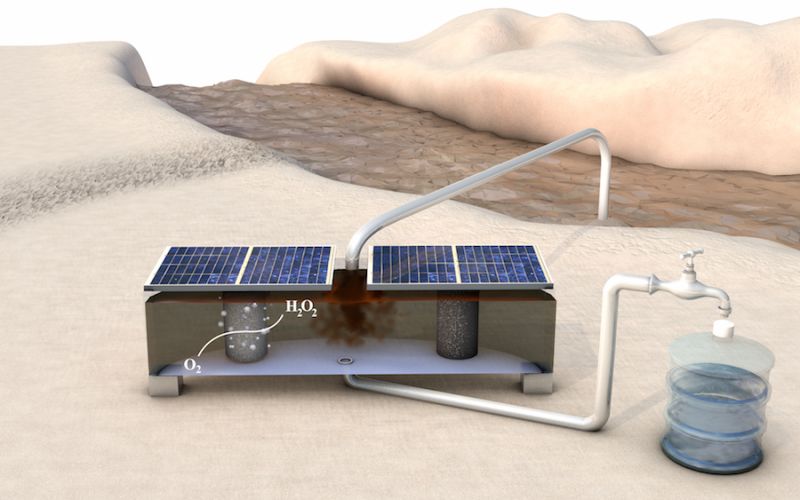
Limited access to clean water is a major issue for billions of people in the developing world, where water sources are often contaminated with urban, industrial and agricultural waste. Many disease-causing organisms and organic pollutants can be quickly removed from water using hydrogen peroxide without leaving any harmful residual chemicals. However, producing and distributing hydrogen peroxide is a challenge in many parts of the world.
Now scientists at the Department of Energy's SLAC National Accelerator Laboratory and Stanford University have created a small device for hydrogen peroxide production that could be powered by renewable energy sources, like conventional solar panels.
"The idea is to develop an electrochemical cell that generates hydrogen peroxide from oxygen and water on site, and then use that hydrogen peroxide in groundwater to oxidize organic contaminants that are harmful for humans to ingest," said Chris Hahn, a SLAC associate staff scientist.
Their results were reported March 1 in Reaction Chemistry and Engineering.
The project was a collaboration between three research groups at the SUNCAT Center for Interface Science and Catalysis, which is jointly run by SLAC and Stanford University.
"Most of the projects here at SUNCAT follow a similar path," said Zhihua (Bill) Chen, a graduate student in the group of Tom Jaramillo, an associate professor at SLAC and Stanford. "They start from predictions based on theory, move to catalyst development and eventually produce a prototype device with a practical application."

In this case, researchers in the theory group led by SLAC/Stanford Professor Jens Nørskov used computational modeling, at the atomic scale, to investigate carbon-based catalysts capable of lowering the cost and increasing the efficiency of hydrogen peroxide production. Their study revealed that most defects in these materials are naturally selective for generating hydrogen peroxide, and some are also highly active. Since defects can be naturally formed in the carbon-based materials during the growth process, the key finding was to make a material with as many defects as possible.
"My previous catalyst for this reaction used platinum, which is too expensive for decentralized water purification," said research engineer Samira Siahrostami. "The beautiful thing about our cheaper carbon-based material is that it has a huge number of defects that are active sites for catalyzing hydrogen peroxide production."
Stanford graduate student Shucheng Chen, who works with Stanford Professor Zhenan Bao, then prepared the carbon catalysts and measured their properties. With the help of SSRL staff scientists Dennis Nordlund and Dimosthenis Sokaras, these catalysts were also characterized using X-rays at SLAC's Stanford Synchrotron Radiation Lightsource (SSRL), a DOE Office of Science User Facility.
"We depended on our experiments at SSRL to better understand our material's structure and check that it had the right kinds of defects," Shucheng Chen said.
Finally, he passed the catalyst along to his roommate Bill Chen, who designed, built and tested their device.
"Our device has three compartments," Bill Chen explained. "In the first chamber, oxygen gas flows through the chamber, interfaces with the catalyst made by Shucheng and is reduced into hydrogen peroxide. The hydrogen peroxide then enters the middle chamber, where it is stored in a solution." In a third chamber, another catalyst converts water into oxygen gas, and the cycle starts over.
Separating the two catalysts with a middle chamber makes the device cheaper, simpler and more robust than separating them with a standard semi-permeable membrane, which can be attacked and degraded by the hydrogen peroxide.

The device can also run on renewable energy sources available in villages. The electrochemical cell is essentially an electrical circuit that operates with a small voltage applied across it. The reaction in chamber one puts electrons into oxygen to make hydrogen peroxide, which is balanced by a counter reaction in chamber three that takes electrons from water to make oxygen—matching the current and completing the circuit. Since the device requires only about 1.7 volts applied between the catalysts, it can run on a battery or two standard solar panels.
The research groups are now working on a higher-capacity device.
Currently the middle chamber holds only about 10 microliters of hydrogen peroxide; they want to make it bigger. They're also trying to continuously circulate the liquid in the middle chamber to rapidly pump hydrogen peroxide out, so the size of the storage chamber no longer limits production.
They would also like to make hydrogen peroxide in higher concentrations. However, only a few milligrams are needed to treat one liter of water, and the current prototype already produces a sufficient concentration, which is one-tenth the concentration of the hydrogen peroxide that you buy at the store for your basic medical needs.
In the long term, the team wants to change the alkaline environment inside the cell to a neutral one that's more like water. This would make it easier for people to use, because the hydrogen peroxide could be mixed with drinking water directly without having to neutralize it first.
The team members are excited about their results and feel they are on the right track to developing a practical device.
"Currently it's just a prototype, but I personally think it will shine in the area of decentralized water purification for the developing world," said Bill Chen. "It's like a magic box. I hope it can become a reality."
More information: Zhihua Chen et al. Development of a reactor with carbon catalysts for modular-scale, low-cost electrochemical generation of HO, React. Chem. Eng. (2017). DOI: 10.1039/C6RE00195E
Provided by SLAC National Accelerator Laboratory




















