Heat treatment offers precise control over catalytic activity of metal sulfide nanoparticles
Nanoparticle catalysts developed by A*STAR researchers can help split water to produce hydrogen, a clean-burning fuel that provides a convenient way to store renewable energy.
Platinum is currently the most efficient catalytic electrode material for generating hydrogen in this way, but the precious metal is both scarce and expensive. Yee-Fun Lim and colleagues at the A*STAR Institute of Materials Research and Engineering have now developed electrocatalyst nanoparticles that are highly active, cheap and stable, and which perform the hydrogen evolution reaction as well as any alternatives to platinum yet discovered.
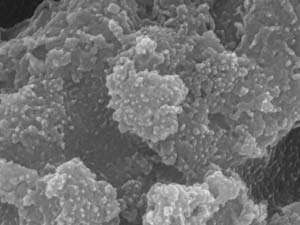
The team used porous nickel foam as the basis for their electrode, because it provides a very large surface area to support active catalytic nanoparticles. Then they coated the foam with a cobalt-thiourea compound, and heated it to break down the thiourea, which released sulfur. This sulfur reacted with the metals to form nanoparticles of cobalt sulfide and nickel sulfide. The researchers studied the structure and composition of the nanoparticles using a variety of techniques, including X-ray diffraction and scanning electron microscopy.
During the reaction, electricity helps metal atoms on the surface of these nanoparticles to pluck a hydrogen atom from a water molecule. The hydrogen atom then combines with another hydrogen atom—either on the nanoparticle's surface, or from another water molecule—to make hydrogen gas (H2). Crucially, the metal sulfide nanoparticles operate well under the alkaline conditions usually required for the parallel reaction that generates oxygen during water splitting.
Lim's team showed that varying the temperature and duration of the heating step used to prepare the nanoparticles had a dramatic effect on their composition and relative proportions, and tests showed that this determined their activity in the hydrogen evolution reaction. Prolonged heating caused some of the nanoparticles to clump together, for example, and also increased the proportion of cobalt sulfide, which significantly reduced the catalyst's activity.
The best performance came from the mixed-metal sulfide that had been heated to 500 degrees Celsius for just 10 minutes (see image). It required a relatively low voltage of 163 millivolts to initiate the hydrogen evolution reaction, just 47 millivolts higher than a commercial platinum electrocatalyst, and comparable to the best alternatives. The catalyst showed no degradation over three days of continuous reactions.
"The mixed catalyst combines the good properties of both nickel and cobalt catalysts to achieve superior performance," says Lim. His team plans to use a similar approach to tailor-make catalytic nanoparticles for a different reaction that turns carbon dioxide into fuels.
More information: Davide Ansovini et al. A highly active hydrogen evolution electrocatalyst based on a cobalt–nickel sulfide composite electrode, J. Mater. Chem. A (2016). DOI: 10.1039/c6ta00540c
Journal information: Journal of Materials Chemistry A





















