Re-energizing the lithium-ion battery
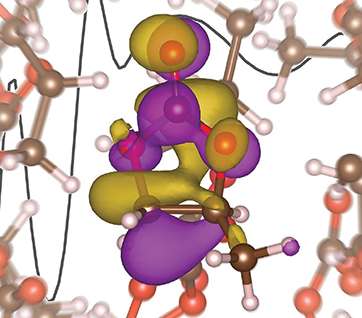
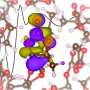
High costs, slow recharging rates, and limited lifetimes restrict the utility of lithium-ion batteries for electric vehicles, storing electricity from wind or solar power, and other applications. Scientists are resolving these deficiencies; however, few have focused on a key interaction that influences battery behavior—how the lithium ions move from one electrode to the other. Researchers at the Lawrence Berkeley National Laboratory and the University of California-Berkeley have taken up the challenge. Using experiments and theoretical calculations, they showed that the lithium ion's journey involves more intimate contact with the electrolyte molecules than previously thought.
These findings suggest that computational models need to be refined to account for the higher number of electrolyte molecules surrounding the lithium ion (its solvation structure) when representing the lithium ion-electrolyte interaction. The improved modeling of the lithium ion solvation structure could allow lithium-ion batteries to take on new applications.
In recent years, lithium-ion batteries have saturated the electronics market due to their widespread use in cell phones, laptop computers, and tablets. As they comprise such a crucial aspect of modern technology, billions of dollars have been spent to maximize the usefulness of the lithium-ion battery. Yet shortcomings, such as high cost, slow recharging rates, and limited lifetimes, restrict the utility of these batteries. Various aspects of the lithium-ion battery have been targeted by research seeking to remedy these deficiencies; however, little effort has been focused on discerning exactly how the lithium ions move from one electrode to the other.
Researchers at the Lawrence Berkeley National Laboratory and the University of California-Berkeley have honed in on this very aspect by investigating the detailed solvation structure of the lithium ion. Using liquid microjets to measure x-ray absorption spectra, interpreted using first-principles theory calculations, this group has determined that the lithium ion has a solvation number of 4.5, which varies from the expected tetrahedral structure. These findings suggest that future computational models should expand beyond the current tetrahedral model to improve upon the electrolytes within the battery. The improvement of the lithium-ion battery based on these findings could be another step towards making the batteries even more useful for large-scale applications.
More information: Jacob W. Smith et al. X-Ray absorption spectroscopy of LiBFin propylene carbonate: a model lithium ion battery electrolyte, Phys. Chem. Chem. Phys. (2014). DOI: 10.1039/C4CP03240C
Provided by US Department of Energy