Changing pluripotent stem cells to differentiated cells
Stem cells are an effective tool for repairing or replacing damaged or diseased tissues, but only if they can be reliably developed from their flexible 'pluripotent' state into a mature 'differentiated' state. A*STAR researchers have learned how to control the state of stem cells by altering the physical environment in which they are cultured.
Chemical signals and mechanical forces help determine which cells differentiate and which remain pluripotent. Researchers have gained several insights into the chemical 'cues', but are still struggling to understand how to manipulate the structure of stem cell colonies in order to control their behavior. "There has been a lot of trial and error because of the lack of engineering principles to guide the control of cellular responses," says Hanry Yu of the A*STAR Institute of Bioengineering and Nanotechnology. His team focused on E-cadherin and integrin, two cellular adhesion proteins that are believed to be involved in regulating stem cell development. Intriguingly, both proteins act via a common signaling factor, Rho-ROCK-myosin II, raising questions about how they induce distinct cellular fates.
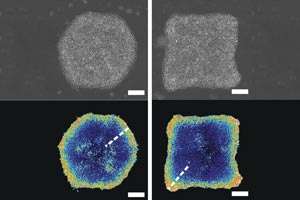
Initial experiments showed that cultured stem cells at the boundaries of colonies were more prone to undergo differentiation, supporting a previously proposed model in which stress-induced integrin signals at the edges actively promote differentiation (see image). However, further studies with cells cultivated on surfaces coated with patterns of these two proteins demonstrated that E-cadherin is actually the dominant signal, with the capacity to override integrin and compel stem cells to remain pluripotent. "At the colony center, E-cadherin-mediated strong cell-cell interaction inhibited stem cell differentiation," says Yu. "Near the edge, the relatively less packed cells and higher stress caused a reduction in E-cadherin-mediated interaction that released the block on differentiation." This process appears to be mediated in part by the preferential localization of Rho-ROCK-myosin II at sites of E-cadherin interaction, which prevents it from interacting with integrin.
These findings could help clinical researchers to strongly control the population-scale behavior of stem cell cultures, maintaining them in a fully pluripotent or differentiated state. "It is difficult and costly to use impure cell sources for wound repair or regenerative medicine applications," says Yu. In the future, he plans to extend the 'micropatterning' approach applied here to achieve even more precise modulation of stem cell behavior. "We plan to hijack the signaling process with soluble or patterned inhibitors to trigger controlled differentiation of human embryonic stem cells," says Yu.
More information: Yi-Chin Toh et al. Modulation of integrin and E-cadherin-mediated adhesions to spatially control heterogeneity in human pluripotent stem cell differentiation, Biomaterials (2015). DOI: 10.1016/j.biomaterials.2015.01.019
Journal information: Biomaterials