Controls of specialization unraveled

Two phases of the cell cycle of human embryonic stem cells have been shown, for the first time, to actively employ pathways that maintain pluripotency—the potential to develop into almost any type of cell in the body.
Embryonic stem cells are derived from a group of cells present in an embryo before its implantation in the womb. For differentiation of these cells there must be a breakdown of the processes that maintain pluripotency. Several studies have identified pathways that regulate the cessation of mouse embryonic stem cells' pluripotency, however the process for human embryonic stem cells (HESCs) has remained a mystery.
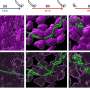
Researchers at A*STAR's Genome Institute of Singapore used RNA to 'knock down' specific genes in HESCs, a method called high-throughput RNA interference screening. By using this method under a variety of conditions, they could identify pathways that regulate the exit of HESCs from pluripotency into specialized differentiation.
"We found a lot of different pathways, but I think the most exciting one was the involvement of the cell cycle," says cell biologist, Kevin Gonzales.
The previous dogma, explains Gonzales, was that the G1 phase—the first of four phases of cell division—was the only part of the cell cycle actively regulating pluripotency. The G1 phase is known to receive signals and express factors that encourage the differentiation of HESCs into specialized cells.
However, the A*STAR team found that the subsequent S and G2 phases of the cell cycle also have particular pathways that maintain pluripotency. In fact, the researchers believe that the absence of these pathways in the G1 phase make this stage more responsive to cues that induce cell differentiation.
The team also found that HESCs tend to maintain pluripotency, rather than proceed to differentiation, when their DNA is damaged. HESCs can better repair DNA damage when they are pluripotent. If repair fails, the cells enter a death pathway: an easier process when they are pluripotent than when differentiating. This status means differentiating cells will not give rise to damaged cells, says Gonzales, essential in the context of an embryo because it prevents the production of a large number of cells in the body with DNA damage.
This study was basic research, rather than having a particular application, says Gonzales. But these new understandings are significant as they can help to fine-tune laboratory protocols on how to control the differentiation of HESCs, he says.
More information: Uy Gonzales et al. Deterministic Restriction on Pluripotent State Dissolution by Cell-Cycle Pathways, Cell (2015). DOI: 10.1016/j.cell.2015.07.001
Journal information: Cell