Architecture of a lipid transport protein revealed
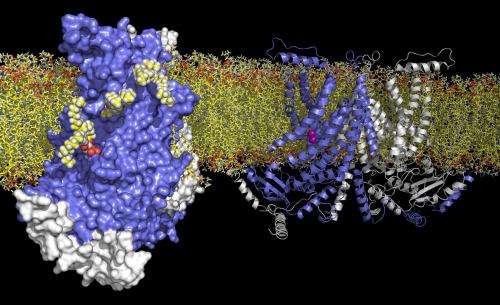
For the first time, the complex architecture of a protein that controls the transport of lipids between the two layers of a cell membrane has been described. With this structure, Biochemists from the University of Zurich have now gained insight into processes that trigger blood coagulation.
Membranes are thin walls that surround cells and protect their interior from the environment. These walls are composed of phospholipids, which, due to their amphiphilic nature, form bilayers with distinct chemical properties: While the outward-facing headgroups are charged, the core of the bilayer is hydrophobic, which prevents charged molecules from passing through. The controlled flow of ions across the membrane, which is essential for the transmission of nerve impulses, is facilitated by ion channels, membrane proteins that provide gated pathways for ions. Analogous to ion channels, lipid scramblases facilitate the passage of phospholipids beween the two layers of a membrane, a process that plays a key role in the intitiation of blood coagulation. Until recently, however, the architecture of these lipid scramblases remained unknown
Now, for the first time, researchers from the Department of Biochemistry of the University of Zurich, have succeeded in the structure determination of a lipid scramblase. A team of scientists in the group of Professor Raimund Dutzler unveiled the structure of a lipid scramblase from the TMEM16 family by X-ray crystallography. The structure provides insight into the activation of the protein by calcium and the transport of lipids. The work has now been published in the scientific journal Nature.
The architecture of a new membrane protein family
Membrane proteins of the TMEM16 family show a unique functional breadth, since they include, besides ion channels, which are essential for regulating of smooth muscle contraction, olfaction and eptithelial chloride secretion, also proteins that act as lipid scramblases. When activated by calcium, these lipid scramblases located in the plasma membrane of blood platelets trigger blood coagulation by facilitating the transport of the lipid phosphatidylserine to the surface of the cell. In order to understand this process, the researchers have characterized the structure and function of a closely related fungal TMEM16 lipid scramblase. Their work has revealed a novel protein architecture that is common to the entire family and offers insight into lipid transport.
"The protein contains a charged crevice, which traverses the membrane in the form of a spiral staircase. This allows the polar headgroup of lipids to move from one side of the membrane to the other," explains first author Janine Brunner. In the vicinity of this crevice, there are bound calcium ions surrounded by conserved, negatively charged side chains. Mutations in the calcium binding site impair lipid transport. By studying the calcium dependence of channel activation in the related TMEM16 chloride channels by electrophysiology, the scientists demonstrated the conservation of this calcium binding mode within the TMEM16 family.
Basis for new therapies
The results form the basis for understanding previously unknown mechanisms of lipid transport. "We have now gained insight into the architecture and function of a family of proteins, the malfunctioning of which causes various hereditary diseases," says the biochemist from UZH. The modulation of these proteins by specific drugs could be a potential strategy for novel therapies – such as the treatment of Scotts syndrome, a blood coagulation disorder, or of a muscle disease associated with the malfunctioning of TMEM16 proteins.
More information: Janine D. Brunner, Novandy. K. Lim, Stephan Schenck, Alessia Duerst and Raimund Dutzler. "X-ray structure of a calcium-activated TMEM16 lipid scramblase." Nature. November 12, 2014. DOI: 10.1038/nature13984
Journal information: Nature
Provided by University of Zurich