Scientists identify key factor that maintains stem cell identity

A protein implicated in several cancers appears to play a pivotal role in keeping stem cells in an immature "pluripotent" state, according to a new study by NYU Langone Medical Center scientists. The study is published online today in Cell Reports.
Stem cells are the perpetual adolescents of the cellular world, uncommitted to any cell fate. In principle, they can be programmed to differentiate into any mature cell type, holding the promise of regenerating tissues and organs. A fuller understanding of their biology, however, is needed.
"Our finding provides a better understanding of the complexity of how the stem cell state is regulated," says Eva M. Hernando-Monge, PhD, associate professor of pathology and a member of the Helen L. and Martin S. Kimmel Center for Stem Cell Biology at NYU Langone Medical Center.
The newly identified stem cell factor is BRD4, a protein associated with several cancers and the target of prospective therapies currently in clinical trials. In 2013, Dr. Hernando-Monge and colleagues found that BRD4 is overexpressed in melanoma cells and helps sustain their proliferation, whereas inhibiting BRD4 greatly slows their growth. The protein appeared to drive cancer in part by keeping cancer cells in a relatively immature, stem cell-like state. Intrigued, Dr. Hernand-Monge wanted to find out what role the protein played in actual stem cells.
In the new study, Dr. Hernando-Monge's team inhibited BRD4's activity in mouse and human embryonic stem cells using BRD4-blocking compounds developed by collaborator Ming-Ming Zhou and colleagues at the Icahn School of Medicine at Mount Sinai. They also used special RNA molecules that block BRD4 gene transcripts, and observed the cells shift out of the stem cell state. As they divided, the cells began to show characteristics of young neurons. Stem cells are thought to maintain a state of quiescence until some signal forces them to divide, producing a differentiated, highly specialized cell.
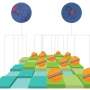
BRD4 has been known to regulate gene activity by binding to the support structure of DNA, called chromatin, at special switch sites called super-enhancers distributed throughout the genome. These sites are believed to be top-level controllers, orchestrating the distinctive expression patterns of several genes that together determine specific cell types such as nerve or muscle.
"We found that BRD4 occupies the super-enhancer sites of genes that are important for maintaining stem cell identity," says Raffaella Di Micco, PhD, a postdoctoral fellow who conceived the research project with Dr. Hernando-Monge and performed most of the experiments. These genes, including OCT4 and PRDM14, showed steep drops in expression when Dr. Di Micco applied BRD4 inhibitors to stem cells.
"OCT4 also represses neuronal differentiation, so we think that the loss of that repression with BRD4 inhibition is the most likely reason for the induction of neuronal characteristics in the stem cells," says Dr. Di Micco.
OCT4 is also one of the four factors in the standard "OKSM" cocktail used for turning ordinary cells into induced pluripotent stem cells (iPSCs). The new findings suggest that BRD4 enforces stem cell identity from an even higher regulatory level in the cell. "In theory we could replace one or more of those OKSM factors with BRD4, or add it to the cocktail to increase reprogramming efficiency—that's something we're working on now," says Dr. Hernando-Monge.
Conversely, she notes, BRD4 inhibitors could be used to help program cells in the other direction, turning stem cells into baby neurons, for example, which perhaps one day would be used for regenerative therapies.
Journal information: Cell Reports
Provided by New York University School of Medicine