Filming bacterial life in multicolor as a new diagnostic and antibiotic discovery tool
(Phys.org)—An international team of scientists led by Indiana University chemist Michael S. VanNieuwenhze and biologist Yves Brun has discovered a revolutionary new method for coloring the cell wall of bacterial cells to determine how they grow, in turn providing a new, much-needed tool for the development of new antibiotics.
Discovery of the new method is expected to broadly impact both basic and applied research tied to understanding, controlling, or preventing bacterial cell growth in specific environments, said the two scientists in IU Bloomington's College of Arts and Sciences.
"Understanding the mechanisms controlling bacterial cell growth and shape is of tremendous importance in any area where we seek strategies for controlling bacteria, be it for the eradication of pathogens from the human body or the improvement of bacterial growth in bioremediation and industrial processes," VanNieuwenhze said. "Now, with the development of this one-step method to identify the zones of growth in bacterial cells, we have a dramatically improved toolkit to understand the basic mechanisms of bacterial growth that will directly enable the development of antibacterial strategies."
The paper, "In situ Probing of Newly Synthesized Peptidoglycan in Live Bacteria with Fluorescent D-Amino Acids," will be published in an upcoming edition of Angewandte Chemie, the journal of the German Chemical Society and one of the highest-ranked chemistry-specific journals of original research in the world.
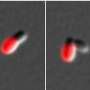
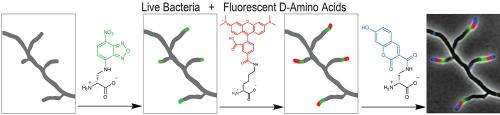
In the paper the authors describe the first direct and universal approach for labeling peptidoglycan, the mesh-like polymer of unusual peptides and sugars that form the cell wall in diverse bacteria. The new method exploits the tolerance of cells for incorporating unnatural D-amino acid-based fluorescent dyes of various sizes and functionalities. The researchers found that these non-toxic dyes preferentially label the sites where the peptidoglycan is synthesized, enabling fine spatiotemporal tracking of cell wall dynamics.
"This method will also enhance our understanding of how bacterial growth is influenced by environmental changes, for example during the development of the human body or as a result of pollution in an environment," Brun said. "Until now, there have been limited ways to visualize active sites of cell growth, and no methods to assess microbial activity exactly where it occurs. Here we have a rapid, simple, and universal strategy for direct observation of when and where living bacteria build their cell wall. I like to use Steve Jobs' famous quote when describing this method to my colleagues: 'It just works!' "
"We have synthesized dyes of different colors that we can use to see what part of the cell grew at different times," said graduate student Erkin Kuru, the lead author of the paper. "If we add dyes of different colors at different times during bacterial growth, the cell wall acts almost like a tape recorder for the morphological changes the cells go through during the respective time of exposure to the dyes. As a result, the final picture of the multicolored bacterial cell tells us what part of the cell grew and by how much at the respective time of exposure to the dyes. It's like making a movie of the life of a cell in multicolor."
The researchers found that the new dyes seem to work with any bacterial species, making them a powerful tool for uncovering how a variety of bacteria grow. The new reagents are also expected to allow scientists to make very selective modifications to bacterial cell surfaces that have different functions, in turn allowing for the development of a battery of new diagnostic and therapeutic probes. Furthermore, the affinity of bacteria for unnatural D-amino acids is also expected to pave the way for design and synthesis of novel D-amino acid-based antibacterials.
"Cell wall synthesis is the major target of current antibiotics," VanNieuwenhze said. "There has always been an arms race with bacteria because they constantly develop resistance to antibiotics, so new ones are always needed. We see this as a powerful new tool in that arms race because the cell wall is an excellent target for antibiotics."
More information: VanNieuwenhze, A. In Situ Probing of Newly Synthesized Peptidoglycan in Live Bacteria with Fluorescent D-Amino Acids, Angewandte Chemie International Edition, dx.doi.org/10.1002/anie.201206749
Journal information: Angewandte Chemie
Provided by Indiana University