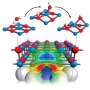
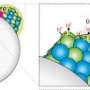
Novel method yields highly reactive, highly hydroxylated TiO2 surface

(PhysOrg.com) -- Build a surface of titanium and oxygen atoms arranged just so, coat with water, and add sunshine. What do you get? In theory, energy-rich hydrogen produced by photolysis—a process by which water molecules placed on a catalytic surface and exposed to electromagnetic radiation are split, forming H2 and O2. In reality, that hydrogen is getting closer to our grasp.
A novel method created by an EMSL user team for producing a highly reactive titania (TiO2) surface with every other oxygen atom converted to a hydroxyl (OH) group, as well as an atomic-level characterization of the surface structure and reactivity, is featured on the cover of the March 7, 2012 issue of PCCP.
The team’s new method to make hydroxyl-rich TiO2 is a two-step photochemical process that involves first adsorption then exposure to ultraviolet light. It holds sway over other synthesis routes because of its high yield of surface OH groups and its reproducibility. Further, the team integrated scanning tunneling microscopy (STM), temperature programmed desorption, and photo-stimulated desorption techniques at EMSL as well as density functional theory calculations performed at both EMSL and the National Energy Research Scientific Computing Center to fully understand the structure and reactivity of their surface. Arguably, the researchers’ most important finding was that upon annealing to elevated temperatures no hydrogen atoms on the surface diffused into the TiO2 bulk to become unwanted dopants or charge traps that would affect the performance.
Insights afforded by this work from EMSL, Pacific Northwest National Laboratory, and Worcester Polytechnic Institute scientists have implications for better understanding how to tailor TiO2 thin films for optimal performance in a myriad of applications, not least of which is the generation of clean, renewable energy by water splitting.
More information: Du Y, et al. 2012. “Hydrogen reactivity on highly-hydroxylated TiO2(110) surfaces prepared via carboxylic acid adsorption and photolysis.” Physical Chemistry Chemical Physics 14(9):3066–3074. DOI: 10.1039/c1cp22515d
Journal information: Physical Chemistry Chemical Physics
Provided by Environmental Molecular Sciences Laboratory