Harnessing sunlight with semiconductor microspheres containing metal nanoparticles
A*STAR researchers have performed theoretical calculations to explain why semiconductor microspheres embedded with metal nanoparticles are so good at using sunlight to catalyze reactions.
Photocatalysts accelerate chemical reactions by absorbing light from the sun and using the energy to drive reactions on their surfaces. They are attractive for environmentally friendly applications such as generating hydrogen from water and breaking down pollutants. Experimental studies have shown that microspheres made from metal-oxide semiconductors and embedded with metal nanoparticles are particularly effective photocatalysts, but researchers have been uncertain about why this was the case.
Now, Ping Bai and his colleagues at the A*STAR Institute of High Performance Computing in Singapore have performed computer simulations that reveal what makes these structures such effective photocatalysts. Their study also provides scientists with helpful guidelines for designing plasmonic photocatalysts.
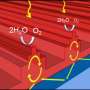
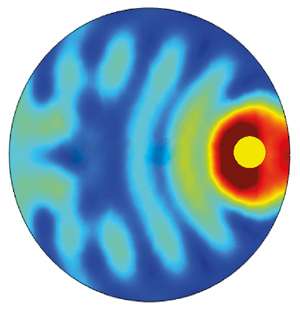
Bai and his colleagues used a widely employed computational technique known as the finite element method to analyze how light interacts with a semiconductor microparticle containing a single metal nanoparticle. Their analysis revealed that the refractive index difference between the semiconductor and the catalytic medium sets up an interference pattern within the semiconductor microparticle. This interference enhances the light absorption of the embedded metal nanoparticles as a result of plasmon resonance (see image).
As a consequence, the microspheres with embedded metal nanoparticles drive chemical reactions by harnessing solar energy much more efficiently than other commonly used photocatalyst structures. "The broadband absorption enhancement exists everywhere inside the microspheres," explains Bai, "and the maximum enhancement can be hundred times greater than that of metal nanoparticles or small core–shell photocatalysts." This explains their superior catalytic rates measured in previous experiments.
In addition to explaining previous experimental findings, the analysis can also be used to inform the design of photocatalysts. In particular, it suggests that using semiconductors with higher refractive indices will maximize the broadband absorption induced by the interference, while using a mix of different plasmonic nanoparticles will enable flexible energy harvesting and enhanced selectivity. Finally, the findings also imply that locating the metal nanoparticles close to the surfaces of the microspheres will increase the catalytic rate as a consequence of the very short range of the plasmon near field.
Bai and his team are now seeking to join forces with others working in the field. "Our next step is to look for end users and experimental collaborators to design, optimize and fabricate particular photocatalysts," says Bai.
More information: "Interference-induced broadband absorption enhancement for plasmonic-metal@semiconductor microsphere as visible light photocatalyst." ACS Catalysis 4, 4269–4276 (2014). dx.doi.org/10.1021/cs501399a
Journal information: ACS Catalysis