Chemists challenge conventional understanding of how photocatalysis works
Photocatalysis—catalysis assisted by light—is a promising route to convert solar energy into chemical fuels. Particularly appealing is the possibility to use photocatalysis to split water molecules into molecular hydrogen. Although photocatalysis has been around for many years, the search for viable photocatalysts to facilitate the splitting of water molecules continues to date.
Photocatalysts are most often semiconductors, with metals such as platinum or gold added to promote their activity. However, these metals (or "promoters") are expensive. There is a need, therefore, to find more economical alternatives.
Now a team of chemists at the University of California, Riverside has come up with a model to explain this promoting effect that could shift the focus in the search for substitutes of the metals and help identify better promoters for photocatalysis in the near future.
Study results appear online this week in the Proceedings of the National Academy of Sciences.
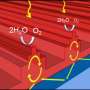
The conventional understanding is that the production of hydrogen from water is promoted due to a fast transfer of excited electrons from the semiconductor to the metal. The UC Riverside researchers report experimental evidence that challenges this well-established explanation.
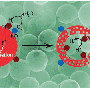
They note in their paper that electron transfer from the semiconductor to the metal may not play a significant role in photocatalysis. Instead, their data support a model where the excited electron promotes the reduction of hydrogen atoms on the surface of the semiconductor, not the metal, and where the reduced atomic hydrogen then migrates from the semiconductor to the metal to recombine and yield molecular hydrogen. In effect, the metal acts as a regular catalyst for the recombination of hydrogen atoms.
"The idea that the function of the metal is to act as a chemical rather than an electronic agent, by recombining hydrogen atoms rather than trapping electrons, is somewhat radical, and has not been proposed before—as far as we know," said Francisco Zaera, a distinguished professor of chemistry, who led the research. "Our results lead us to argue that what is needed is a good atomic hydrogen recombination catalyst."
Hydrogen is arguably the fuel of the future. Like natural gas or oil, it can be burned to generate large amounts of heat and energy. However, its combustion is clean, producing only water. Although handling of hydrogen, a gas, presents some challenges, it could still be a viable cheap fuel for some applications if good photocatalysts were to be developed.
The model proposed by the UCR team paves the way for identifying better alternatives for photocatalysts used in water splitting.
"Currently, many of the photocatalyst promoters considered are expensive precious metals," Zaera said. "Some scientists are searching for cheaper alternatives, but, in our opinion, perhaps with the wrong premise. In our paper we offer an example of how carbon, which could be a choice based on the conventional model, does not work as a photocatalyst, whereas nickel oxide, which should not work if the conventional model is to be believed, does."
The UCR researchers started working on the research project when Yin's lab made novel yolk-shell nanostructures (with gold nanoparticles inside titania shells) that offered great control of the structural parameters of the samples, something not always easy to do. Once the researchers realized that the photophysical behavior of the titania shells was not affected by the presence of the gold nanoparticle inside, they moved on to study more conventional systems.
"We did not set ourselves to challenge the existing model," Zaera said. "It was only when the photophysical measurements yielded unexpected results that we began working on coming up with an alternative mechanism."
Already, the team is working on further understanding the basics of the chemistry involved in photocatalysis, returning to the use of the yolk-shell nanostructures to secure a better handle on the structural parameters of the catalyst.
More information: Promotion of atomic hydrogen recombination as an alternative to electron trapping for the role of metals in the photocatalytic production of H2, PNAS, www.pnas.org/cgi/doi/10.1073/pnas.1405365111
Journal information: Proceedings of the National Academy of Sciences
Provided by University of California - Riverside