Iron-reducing bacteria could detoxify chromium

Hexavalent chromium is a major environmental contaminant at several Department of Energy (DOE) sites as well as other sites around the world. Iron-reducing bacteria can convert the oxidized form of iron in clay minerals, called ferric iron, into the reduced form of iron, called ferrous iron, which can then reduce hexavalent chromium to trivalent chromium—the reduced, insoluble and less toxic form of the heavy metal that poses a lower risk of groundwater contamination. This study sheds light on the poorly understood process by which iron-reducing bacteria reduce ferric iron in clay minerals, resulting in ferrous iron that could then immobilize and detoxify chromium.
The findings suggest that the iron-reducing activity of bacteria could be artificially stimulated to produce ferrous iron in clay minerals, which can then reduce hexavalent chromium to trivalent chromium. This promising strategy could potentially enable long-term remediation of heavy metal-contaminated sediments and groundwater aquifers worldwide.
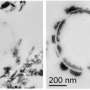
Researchers at Environmental Molecular Sciences Laboratory (EMSL), a DOE national scientific user facility at Pacific Northwest National Laboratory, and Miami University added the iron-reducing bacterium Geobacter sulfurreducensto tubes filled with ferric iron-containing clay minerals such as smectites and chlorite. The researchers used ultra-sensitive microscopy and spectroscopy instruments located in the Quiet Wing, a specialized facility at EMSL, at national scientific user facility at Pacific Northwest National Laboratory. Specifically, they used scanning electron microscopy (SEM) with focused ion beam milling for thinning the samples and performing elemental mapping. They also used transmission electron microscopy (TEM) with electron energy loss spectroscopy for high-resolution imaging and for determining the valence state of chromium and iron before and after the reduction of hexavalent chromium by ferrous iron present in clay minerals.
The bacteria reduced ferric iron in the clay minerals at low rates when they were not stimulated with a compound known as anthraquinone-2,6-disulfonate (AQDS), which enhances their iron-reducing activity. Upon artificial stimulation with AQDS, the bacteria reduced ferric iron present in smectites and smectite-rich clays from DOE's Hanford site, but not chlorite, at significantly higher rates. The resulting ferrous iron in the clay minerals reduced hexavalent chromium at higher rates with increasing temperatures, and at higher rates in smectites compared with chlorite. The observed hexavalent chromium reduction kinetics were well described by a second order rate equation with respect to concentrations of hexavalent chromium and ferrous iron.
SEM and TEM imaging revealed that trivalent chromium was intimately associated with the clay minerals, possibly in the form of sub-nanometer-sized chromium hydroxide embedded in the clay matrix. This structural feature is expected to minimize the secondary contamination risk of trivalent chromium being exposed to environmental oxidants and subsequently converting back to hexavalent chromium. Taken together, the findings suggest that in-situ stimulation of iron-reducing bacteria in iron-bearing clay minerals widely distributed in soils and sediments at contaminated sites may represent a promising strategy to stably immobilize chromium for long-term remediation efforts.
More information: Bishop, M.E., Glasser, P., Dong, H., Arey, B., and Kovarik, L. "Reduction and immobilization of hexavalent chromium by microbially reduced Fe-bearing clay minerals." Geochimica et Cosmochimica Acta. 133, (2014). DOI: 10.1016/j.gca.2014.02.040
Journal information: Geochimica et Cosmochimica Acta
Provided by Environmental Molecular Sciences Laboratory