Iron-bearing minerals in sediments react with and immobilize contaminants

The release of wastes associated with nuclear reprocessing from storage facilities into the underlying sediments and groundwater is an important environmental concern at the Hanford Site. This study provides evidence that iron-bearing minerals naturally abundant in these sediments can react with and immobilize contaminants such as technetium.
This research can be used to predict how long it would take for such reactions to reduce contaminant levels in groundwater. This information is also important for assessing the ability of these natural processes to reduce the risk of groundwater contamination. Moreover, the study suggests that remediation efforts at the Hanford Site could focus on locations lacking iron-bearing minerals capable of reacting with and attenuating contaminants.
During the Cold War era, the Hanford Site in southeastern Washington state produced two-thirds of the plutonium generated in the United States for nuclear weapons. Although wastes associated with nuclear reprocessing have been stored in ponds and underground tanks, contaminants such as uranium, technetium and plutonium have leaked from these storage facilities into the underlying sediments and groundwater. A large amount of effort has been directed to actively address this problem, but the sediments themselves may naturally reduce the migration of some of these contaminants to the groundwater.
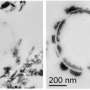
Sediments at the Hanford Site contain magnetic, iron-rich minerals derived from igneous rocks such as basalt. Some of these minerals are iron oxides containing the reduced form of iron, called ferrous iron, which can potentially transfer its extra electron to contaminants and thus stabilize them in an insoluble form in sediments, reducing the risk of groundwater contamination. Researchers from Pacific Northwest National Laboratory (PNNL) and EMSL, the Environmental Molecular Sciences Laboratory, recovered sediments from several locations at the Hanford Site, characterized the iron-bearing minerals, and assessed the ability of these minerals to react with the oxidized, soluble form of technetium, called pertechnetate. They used scanning electron microscopy to characterize particle size and shape, micro-x-ray diffraction to characterize mineral structure, x-ray photoelectron spectroscopy to determine the surface composition of these minerals, and electron probe microanalysis to determine the composition of the bulk sediment, at EMSL, a DOE national scientific user facility.
They found that magnetic minerals made up about one percent of the total weight of the sediment, and an iron oxide called magnetite represented about 90 percent of the total magnetic mineral content. Ferrous iron was located at the surface of the magnetite and thus was accessible for reactions with contaminants in the environment. Moreover, magnetic minerals from the sediments reacted with a solution containing pertechnetate, decreasing levels of this contaminant. Taken together, the findings suggest that ferrous iron transferred electrons to pertechnetate at the mineral surface, producing the reduced, insoluble form of technetium. According to the researchers' calculations, if all of the technecium-99 came into contact with all of the available ferrous iron, this natural process could remove the entire groundwater inventory of this isotope, which poses a major contamination risk at the Hanford site, over a period of a few months.
More information: Pearce, C.I., Liu, J., Baer, D.R., Qafoku, O., Heald, S.M., Arenholz, E., Grosz, A.E., McKinley, J.P., Resch, C.T., Bowden, M.E., Engelhard, M.H., Rosso, K.M. "Characterization of natural titanomagnetites (Fe3−xTixO4) for studying heterogeneous electron transfer to Tc(VII) in the Hanford subsurface." Geochimica et Cosmochimica Acta. (2014). DOI: 10.1016/j.gca.2013.12.010.
Journal information: Geochimica et Cosmochimica Acta
Provided by Environmental Molecular Sciences Laboratory