This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Nitrogen-doped carbon layers boost efficiency and stability of nickel catalysts at room temperature
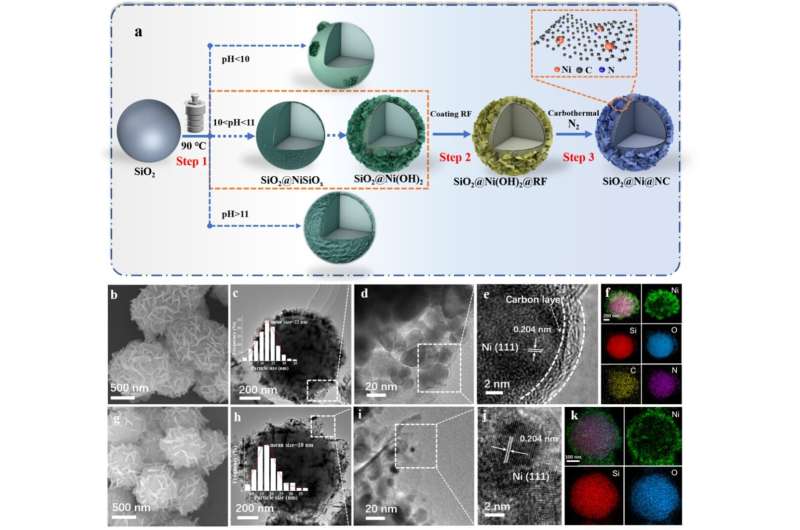
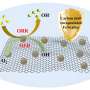
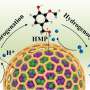
A research team led by Wang Guozhong from the Hefei Institutes of Physical Science of the Chinese Academy of Sciences encapsulated metallic nickel in a nitrogen-doped carbon-silica composite (SiO2@Ni@NC) as a catalyst, which showed good performance in vanillin hydrogenation in aqueous media.
They found that it can achieve 99.8% vanillin conversion and 100% selectivity of 4-hydroxymethyl-2-methoxyphenol (HMP) at room temperature. The results were published in Advanced Science.
Water is an easily accessible and environmentally friendly solvent. However, catalytic reactions involving water are severely limited by difficulties in stabilizing the active metal species. Studies have shown that encapsulation strategies can effectively reduce the loss of active species.
The nitrogen-doped carbon (NC) layer derived from resorcinol-formaldehyde resin can enhance the affinity between the catalyst and gas molecules or organic reactants through its inherent hydrophobic properties, effectively improving the catalytic activity and stability.
In this study, the researchers prepared a nickel catalyst wrapped in a mixture of nitrogen-doped carbon and silica called SiO2@Ni@NC. They used it to convert vanillin into another chemical called 4-hydroxymethyl-2-methoxyphenol (HMP) using water as solvent. This packaged catalyst worked very well. It converted almost all of the vanillin to HMP at room temperature, and it could be reused five times.
The efficient catalytic performance came from the synergistic effect of the active metals, the nitrogen-doped carbon layer, and the silica. The silica helped distribute the catalyst evenly in the water, while the carbon layer protected the metals and promoted the reaction. Tests showed that the carbon layer also helped gather the chemicals needed for the reaction.
Density functional theory calculations confirmed the role of the nitrogen-doped carbon layer in the spontaneous dissociation of H2 and elucidated the catalytic mechanism for the aqueous-phase hydrogenation of vanillin.
The carbon layer encapsulation strategies in this work provide a reference for the construction of efficient and stable aqueous hydrogenation catalysts at room temperature, according to the team.
More information: Zidan Zou et al, Toward High‐Performance Hydrogenation at Room Temperature Through Tailoring Nickel Catalysts Stable in Aqueous Solution, Advanced Science (2024). DOI: 10.1002/advs.202309303
Journal information: Advanced Science
Provided by Chinese Academy of Sciences