Encapsulated NiCo alloy nanoparticles catalyzing HDO reactions
The conversion of abundant biomass and their derivatives into high value-added fuels and chemicals is envisaged as a promising green avenue to reduce our reliance on conventional fossil resources. Among them, the pyrolysis of lignocellulose has become a green and economic means for mass production of bio-oils to substitute fossil fuels. However, due to the rich oxygen contents, the obtained bio-oils possess relatively low energy density and could cause damage to engines. The catalytic hydrodeoxygenation (HDO) has therefore been employed to upgrade bio-oils with high energy density by selectively removing oxygen contents.
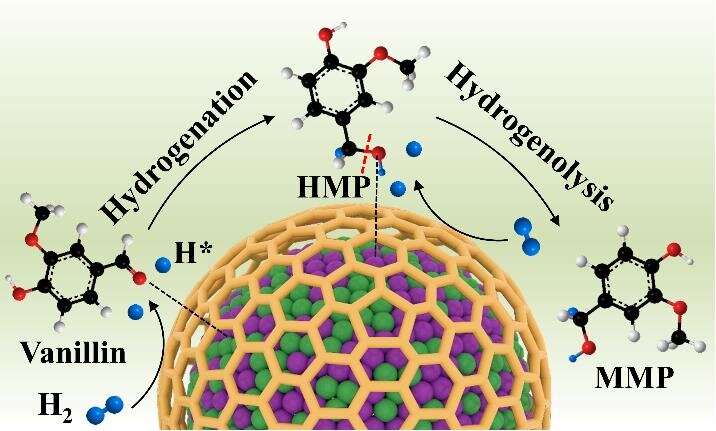
As a direct lignocellulose pyrolysis product, vanillin can be upgraded to 2-methoxy-4-methylphenol (MMP) via HDO. The obtained MMP has wide applications not only as a high energy density fuel but also as an important intermediate to synthesize drugs and fragrances. Therefore, developing efficient nonprecious metal catalysts capable of selectively HDO of vanillin to MMP in water is required.
The synergetic effect of the alloyed nanoparticles (NPs) based catalysts has been advantageously utilized to dramatically enhance the catalyst performance for upgrading biomass and biomass-derivatives to high valued chemicals and fuels. In generally, the group VIII metals (Ni, Ru, Rh, Pd, Pt) possess high catalytic activities for hydrogenation of unsaturated C=C bonds and C=O bonds. For this reason, non-precious Ni-based catalysts have been widely employed to catalyze HDO reactions, but exhibiting limited selectivity toward oxygen-free products. To enhance the deoxygenation activity and selectivity of Ni-based catalysts, allying Ni with other metals to form alloy NPs is an efficient strategy.
Recently, a research team lead by Prof. Huijun Zhao from Institute of Solid State Physics, HFIPS, CAS, China report the controllable synthesis of well-defined Ni-Co alloy NPs confined by N-doped carbon nanotubes (N-CNTs) and their application as an efficient HDO catalyst to transform vanillin, its derivatives and other aromatic aldehydes to the corresponding MMPs and deoxygenated products. The experimental results demonstrate that the as-synthesized Ni-Co alloy NPs catalyst (NiCo@N-CNTs/CMF) can completely convert vanillin to MMP with a 100% selectivity under mild reaction conditions, surpassing the reported high performance nonprecious HDO catalysts. Impressively, the catalyst exhibits excellent HDO catalytic performance toward a wide spectrum of vanillin derivatives and other aromatic aldehydes with 100% conversion efficiency and high selectivity (91.5% to 100%). The DFT calculations and experimental results confirm that the achieved outstanding HDO catalytic performance is due to the greatly promoted selective adsorption and activation of C=O, and desorption of the activated hydrogen species by the synergism of the alloyed Ni-Co NPs. The findings of this work affords a new strategy to design and develop efficient transition metal-based catalysts for HDO reactions in water.
The results were published in Chinese Journal of Catalysis.
More information: Dongdong Wang et al, Encapsulated Ni-Co alloy nanoparticles as efficient catalyst for hydrodeoxygenation of biomass derivatives in water, Chinese Journal of Catalysis (2021). DOI: 10.1016/S1872-2067(21)63828-7
Provided by Chinese Academy of Sciences