This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Chainmail catalysts: Carbon-encapsulated FeNi alloys for enhanced oxygen electrocatalysis
Rapid growth in global energy demand has caused massive depletion of traditional fossil fuels and serious environmental problems, and there is no doubt that the development of efficient energy storage and conversion technologies is an essential field of research. Rechargeable Zn-air batteries have attracted great research interest due to their high energy density, low cost, environmental friendliness, and safety.
However, the sluggish kinetic processes of air cathodes limit the development of Zn-air battery technology, namely the oxygen reduction reaction (ORR) during discharge and the oxygen evolution reaction (OER) during charge. Therefore, efficient electrocatalysts are required to promote these two reactions.
Typically, noble metal-based electrocatalysts such as platinum (Pt) are effective for ORR, while ruthenium (Ru) and iridium (Ir) oxides are effective for OER. However, the unsatisfactory bifunctional catalytic activity, poor stability, low abundance, and high price of noble metal catalysts inevitably hinder the practical application. Therefore, designing efficient and inexpensive catalysts with bifunctional catalytic activity for ORR and OER remains a great challenge.
Over the past decade, researchers have sought to develop bifunctional electrocatalysts without noble metals, including transition metals (Fe, Co, Ni, and Mn), metal alloys, oxides, nitrides, hydroxides, and phosphides. Among these chemicals, transition metal alloys have attracted great interest due to their low price and high catalytic activity for ORR and OER.
In-depth studies have shown that iron–based catalysts can provide excellent catalytic activity for ORR but their OER catalytic performance is poor, while nickel–based catalysts have outstanding performance in OER, and there is no doubt that the combination of Fe and Ni is a wise choice for the construction of efficient bifunctional catalysts.
FeNi alloy electrocatalysts with good ORR and OER catalytic activities simultaneously are highly desirable. There has been some progress in this direction; however, the metal parts still suffer from insufficient durability because repeated redox reactions can lead to metal dissolution in aqueous solutions.
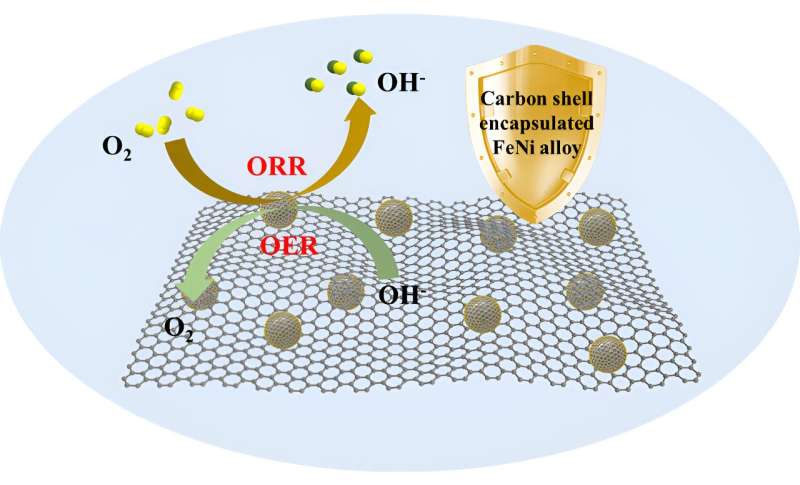
Balancing catalytic activity and durability of alloy electrocatalysts is one of the major challenges in achieving excellent performance. To address this problem, an effective chainmail strategy is to construct an encapsulation structure with carbon materials.
The chemical reaction environment, which typically includes reacting molecules in a liquid solution, temperature, and a variety of physical fields, is like the battlefield on which catalysts fight. The stabilized carbon layer protects the internal metal core from the destructive reaction environment.
It is therefore figuratively described as chainmail catalysts. The chainmail should not only be a robust material for separating and protecting the catalyst from corrosive environments, but should also be able to transfer catalytic activity to its outer surface, which then participates in the catalytic reaction.
Recently, a research team led by Prof. Zhen Zhou from Zhengzhou University, China, designed a highly promising chainmail catalyst named FeNi@NC, comprising ultrathin carbon shells encapsulating FeNi alloy nanoparticles on N-doped graphene-like nanosheets. The strong synergistic effects between FeNi alloys and N-doped carbon shells result in outstanding bifunctional catalytic activity, particularly in alkaline media.
Consequently, Zn-air batteries incorporating FeNi@NC as the catalyst demonstrate exceptional performance, operating reliably at high power density with extended lifespan. Furthermore, computational analyses provided further confirmation of the catalytic activity and revealed that the electron transfer from FeNi alloy nanoparticles to the carbon shells activates the carbon surface, leading to enhanced catalytic performance.
This research not only sheds light on the rational design and synthesis of heteroatom-doped carbon materials supporting the growth-constrained transition metal alloys, but also offers a practical solution for advancing the application of Zn-air batteries.
The research is published in the Chinese Journal of Catalysis.
More information: Yibo Guo et al, Revolutionizing Zn-Air batteries with chainmail catalysts: Ultrathin carbon-encapsulated FeNi alloys on N-doped graphene for enhanced oxygen electrocatalysis, Chinese Journal of Catalysis (2024). DOI: 10.1016/S1872-2067(23)64603-0
Provided by Chinese Academy of Sciences