This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Gut microbiota and antibiotics: Missing puzzle piece discovered
The intricacies of how intestinal bacteria adapt to their environment have yet to be fully explored. Researchers from the Helmholtz Institute for RNA-based Infection Research (HIRI) in Würzburg and the University of California, Berkeley, U.S., have now successfully closed a gap in this knowledge.
They have identified a small ribonucleic acid (sRNA) that affects the susceptibility of the gut pathogen Bacteroides thetaiotaomicron to specific antibiotics. The findings, published today in the journal Nature Microbiology, could serve as the foundation for novel therapies addressing intestinal diseases and combating antibiotic resistance.
The gut, a complex ecosystem of numerous microorganisms, plays a pivotal role in human well-being. Factors like dietary changes, medications, or bile salts can influence the microbiota, impacting health. Among the prevalent intestinal bacteria in humans are Bacteroides thetaiotaomicron. These gut microbes play a role in breaking down polysaccharides during digestion, contributing to human health.
Yet they can also promote infections when the ecosystem is imbalanced, such as after antibiotic treatment. However, the molecular mechanisms enabling these gut microbes to adapt to their environment remain largely unknown.
"My group and I want to understand how Bacteroides thetaiotaomicron adapt to changing conditions in the gut," says Alexander Westermann, summarizing the goal of the study. The initiator of the investigation is a research group leader at the Helmholtz Institute for RNA-based Infection Research (HIRI) in Würzburg, a site of the Braunschweig Helmholtz Centre for Infection Research (HZI) in cooperation with the Julius-Maximilians-Universität Würzburg (JMU).
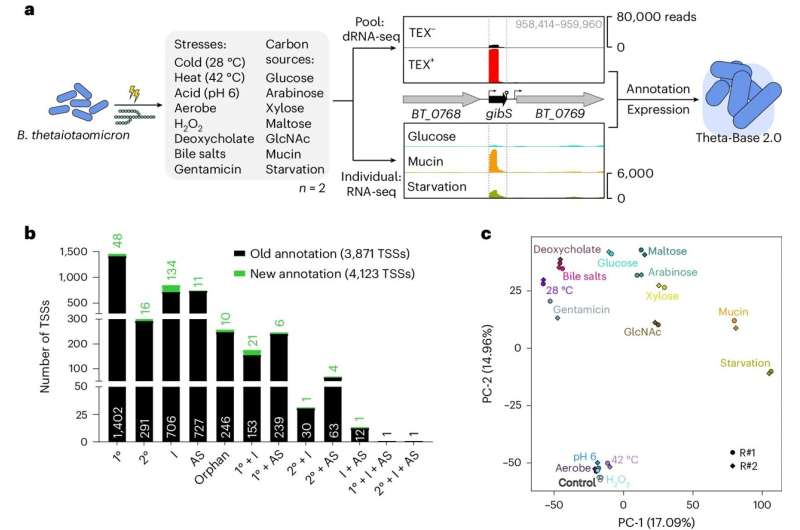
To illuminate the transcription networks in Bacteroides thetaiotaomicron, HIRI scientists collaborated with researchers from the University of California, Berkeley, U.S., to map transcription units and profile their expression under different experimental conditions.
Comparing this gene expression information with literature-derived fitness data from genetically engineered bacterial variants, the research team was able to gain a holistic view of the regulatory networks and the functional role of sRNAs in bacterial adaptation. "Our findings offer new insights into the complex interplay between environmental cues, gene expression, and bacterial fitness," says Westermann, who also holds a professorship at the JMU.
Small RNA regulates antibiotic sensitivity
The researchers, by studying the nucleic acid content of Bacteroides thetaiotaomicron, identified a specific regulatory RNA molecule—a small RNA (sRNA)—that influences the bacterium's sensitivity to tetracycline antibiotics.
"With the discovery of the sRNA MasB and its role in modulating antibiotic sensitivity, we have revealed a previously uncharacterized regulatory mechanism," says first author and postdoctoral researcher Daniel Ryan. The results provide information about the influence of antibiotic treatments on members of the microbiota. On this basis, new strategies for preventing antibiotic-induced collateral damage to beneficial bacteria could be developed and therapeutics improved.
The findings represent a valuable asset for the microbiome community: The comprehensive transcriptome atlas, which is publicly available through the web browser "Theta-Base," presents the opportunity to study more sRNAs and explore their functions in this context.
"The RNA biology of Bacteroides and other members of the gut microbiota is still poorly understood. Research endeavors of this kind lay the groundwork for future investigations, offering a foundational resource for further exploration," says Westermann.
More information: Daniel Ryan et al, An expanded transcriptome atlas for Bacteroides thetaiotaomicron reveals a small RNA that modulates tetracycline sensitivity, Nature Microbiology (2024). DOI: 10.1038/s41564-024-01642-9
Journal information: Nature Microbiology
Provided by Helmholtz Association of German Research Centres