A new form of frozen water? Scientists reveal new ice with record-low density
Amid the season known for transforming Nebraska into an outdoor ice rink, a University of Nebraska-Lincoln-led research team has predicted a new molecular form of the slippery stuff that even Mother Nature has never borne.
The proposed ice, which the researchers describe in a Feb. 12, 2016 study in the journal Science Advances, would be about 25 percent less dense than a record-low form synthesized by a European team in 2014.
If the ice can be synthesized, it would become the 18th known crystalline form of water—and the first discovered in the United States since before World War II.
"We performed a lot of calculations (focused on) whether this is not just a low-density ice, but perhaps the lowest-density ice to date," said Xiao Cheng Zeng, an Ameritas University Professor of chemistry who co-authored the study. "A lot of people are interested in predicting a new ice structure beyond the state of the art."
This newest finding represents the latest in a long line of ice-related research from Zeng, who previously discovered a two-dimensional "Nebraska Ice" that contracts rather than expands when frozen under certain conditions.
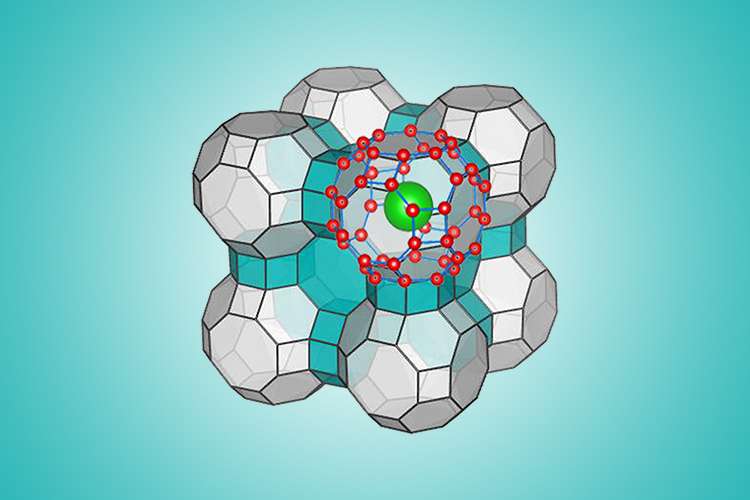
Zeng's newest study, which was co-led by Dalian University of Technology's Jijun Zhao, used a computational algorithm and molecular simulation to determine the ranges of extreme pressure and temperature under which water would freeze into the predicted configuration. That configuration takes the form of a clathrate—essentially a series of water molecules that form an interlocking cage-like structure.

It was long believed that these cages could maintain their structural integrity only when housing "guest molecules" such as methane, which fills an abundance of natural clathrates found on the ocean floor and in permafrost. Like the European team before them, however, Zeng and his colleagues have calculated that their clathrate would retain its stability even after its guest molecules have been evicted.
Actually synthesizing the clathrate will take some effort. Based on the team's calculations, the new ice will form only when water molecules are placed inside an enclosed space that is subjected to ultra-high, outwardly expanding pressure.
Just how much? At minus-10 Fahrenheit, the enclosure would need to be surrounded by expansion pressure about four times greater than what is found at the Pacific Ocean's deepest trench. At minus-460, that pressure would need to be even greater—roughly the same amount experienced by a person shouldering 300 jumbo jets at sea level.
The guest molecules would then need to be extracted via a vacuuming process pioneered by the European team, which Zeng credited with inspiring his own group to conduct the new study.
Yet Zeng said the wonders of ordinary ice—the type that has covered Earth for billions of years—have also motivated his team's research.
"Water and ice are forever interesting because they have such relevance to human beings and life," Zeng said. "If you think about it, the low density of natural ice protects the water below it; if it were denser, water would freeze from the bottom up, and no living species could survive. So Mother Nature's combination is just so perfect."
If confirmed, the new form of ice will be called "Ice XVII," a naming quirk that resulted from scientists terming the first two identified forms "Ice I."
Zeng and Zhao co-authored the Science Advances study with UNL postdoctoral researcher Chongqin Zhu; Yingying Huang, a visiting research fellow from the Dalian University of Technology; and researchers from the Chinese Academy of Sciences and the University of Science and Technology of China.
More information: "A new phase diagram of water under negative pressure: The rise of the lowest-density clathrate s-III" advances.sciencemag.org/content/2/2/e1501010
Journal information: Science Advances
Provided by University of Nebraska-Lincoln