Role model stem cells: How immune cells can self-renew
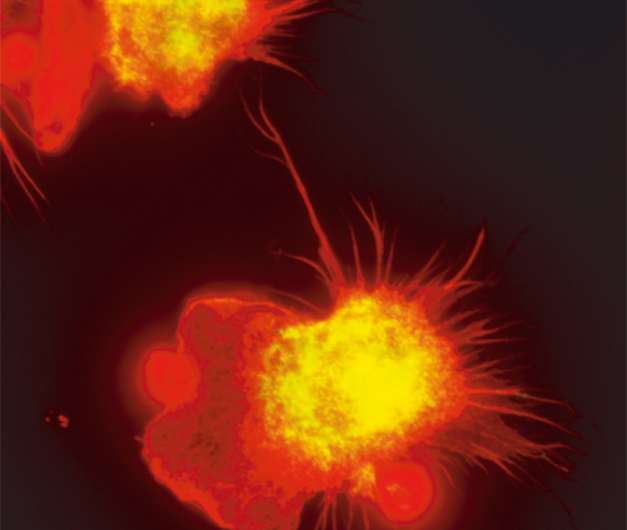
When our organs age or wear out, their renewal usually depends on a few stem cells in the tissue, because the vast majority of differentiated cells have lost their ability to divide and generate new cells. A German-French team led by Michael Sieweke from the Max Delbrück Center for Molecular Medicine (MDC) in Berlin and the Centre d'Immunologie de Marseille-Luminy (CIML) in Marseille has now discovered how human macrophages, a type of specialized immune cell, can nevertheless divide and self-renew almost indefinitely. As the researchers show in the journal Science, the macrophages achieve this by activating a gene network similar to one found in embryonic stem cells. In the future the findings could provide new directions in regenerative medicine and therapies.
Our body is constantly changing: new cells continually replace specialized cells to maintain the skin, intestine, blood, and other tissues or repair them after an injury. Since differentiated cells are usually no longer able to divide, the renewal is almost always accomplished by stem cells specific to the tissue, which are capable of continually generating new cells. Recently researchers have discovered some exceptions: some types of immune cells which have already differentiated possess a capacity for self-renewal.
Macrophages, which play an important role in immune defenses, can also control tissue regeneration and renewal.
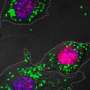
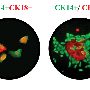
A few years ago, a team led by German immunologist and stem cell researcher Michael Sieweke at the French CIML showed that under certain conditions macrophages can divide without losing features they have acquired while specializing into immune cells. The researchers had shown in mice that proteins that regulate the reading of genes, called transcription factors, play a decisive role in this process. Genetic manipulations that turned off two transcription factors named MafB and c-Maf in the macrophages caused the cells to start what appeared to be a self-renewal program. They could even be maintained and expanded almost indefinitely in cell cultures - which is usually not possible with differentiated cells.
In the new study, Sieweke's research team from the MDC in Berlin and the CIML in France have now been able to show that this also works with various macrophages taken from mice that have not undergone genetic manipulations. This happened when concentrations of both MafB and c-Maf were naturally low or were inhibited for a short time.
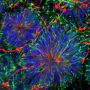
"We now asked ourselves how this is possible - in other words, what mechanisms and genes allow the differentiated macrophages to switch on self-renewal?" Sieweke says. To find out the researchers compared the macrophages to embryonic stem cells, which have a similar, unlimited capacity for self-renewal. The scientists compared the patterns of gene regulatory elements and genes that were active in the two types of cells.
"As it turned out, the macrophages contain a set of dormant genes that can be reawakened and thus enable self-renewal," Sieweke says. In this context, the researchers made a surprising discovery: the macrophage genes work together in a network very similar to one that is switched on in proliferating embryonic stem cells. "You could say that the differentiated cells contain dormant stem cell genes," Sieweke explains.
While the gene networks in the two types of cells are very similar, they are managed in different ways: they are controlled by different transcription factors and gene regulatory elements which are specific to each type of cell. "But it is good news to discover that macrophages can activate the self-renewal genes found in stem cells using their own very specific regulatory factors ," Sieweke says.
He believes that these findings will ultimately be useful in regenerative medicine. "If differentiated cells could be expanded directly, it might be possible to replace diseased tissue without taking a detour via embryonic or induced pluripotent stem cells," Sieweke says. He adds that the dormant gene network may also be activated in other types of cells - mature liver cells, for example, have the ability to divide as well.
Transplantation studies with macrophages suggest that such transplantations of macrophages might indeed be useful for regeneration. Sieweke's teams have already shown that macrophages grown in laboratory cultures do not lose their properties. When injected into mice, the cells successfully re-integrate into tissues and perform all of their normal functions. The cells are not only able to fight infections, but also have an important function in maintaining tissues and are needed for regeneration. "They are, so to speak, the gardeners or guardians of the tissue," Sieweke explains.
More information: "Lineage-specific enhancers activate self-renewal genes in macrophages and embryonic stem cells" dx.doi.org/10.1126/science.aad5510
Journal information: Science
Provided by Helmholtz Association of German Research Centres