Straight up, with a twist: New model derives homochirality from basic life requirements
Life is quirky. Although the molecules that make up all living things obey physical and chemical laws, they do so with a puzzling twist. How did the distinctive molecular features of life emerge, and what can they tell us about life on Earth and elsewhere in the universe?
University of Illinois Swanlund Professor of Physics Nigel Goldenfeld, graduate student Farshid Jafarpour, and postdoctoral researcher Tommaso Biancalani have made a breakthrough in one of the most central chemical quirks of life as we know it: homochirality, the uniform "handedness" of biological molecules. Their new model addressing the emergence of this feature, published in Physical Review Letters and highlighted by Physics suggests that homochirality can be used as a universal signature of life.
All three researchers are members of the Biocomplexity research theme within the Carl R. Woese Institute for Genomic Biology (IGB), and performed their work with funding from the NASA Astrobiology Institute for Universal Biology at UIUC, which Goldenfeld directs.
Many chemicals, organic or otherwise, are chiral; that is, if the structure of each was reflected in a mirror, its "looking-glass" copy could not be turned or flipped to match the original. Like a pair of gloves, the left-handed and right-handed versions of a chiral molecule are functionally equivalent, but their fundamental asymmetry makes them distinct.
Inorganic reactions produce and consume both versions of chiral molecules at equal rates. This is what makes the chirality of biological molecules, such as sugars produced by microbes and plants or the amino acids that make up proteins, so shocking. In every living thing on Earth, all amino acids are left-handed, and all sugars are right-handed. Goldenfeld highlighted the central mystery of this phenomenon.
"Imagine you've got a coin, and it's perfectly made, so it's not biased at all, and you start flipping the coin. Each time you flip it, it keeps coming up heads," he said. "So then you say, something must be operating that's causing this to happen . . . you get the same puzzle with these biological molecules, and that's the problem of homochirality."
Many scientists have proposed hypotheses for how this remarkable asymmetry became dominant. Perhaps the most prominent, put forward by noted physicist Sir Charles Frank in 1953, argued that homochirality could be produced by one of the fundamental properties of life—autocatalysis, the ability to self-replicate. He argued that in a system where one left-handed or right-handed molecule begets more like itself, and each type inhibits the self-replication of the other, an initial unevenness in the ratio, appearing by chance, would ultimately allow one handedness to completely outcompete the other.
Frank's work was ground-breaking, but it left unanswered questions that no subsequent work has adequately addressed. His idea appeared to rely on the inhibition of self-replication of each chirality by the other, a mechanism that might not have existed early on in life's history.
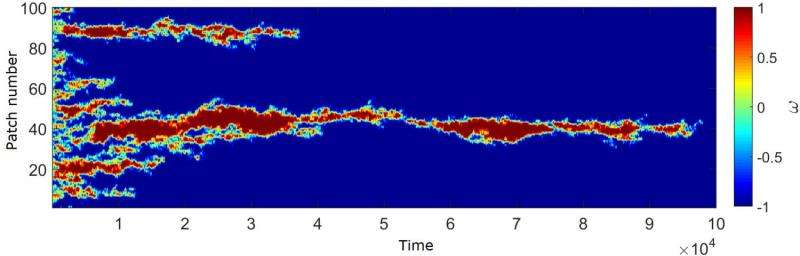
The Illinois team wanted to develop a simpler model, one based on only the most basic properties of life: self-replication and disequilibrium. They showed that with only these minimal requirements, homochirality appears when self-replication is efficient enough.
"There are other models, and they may be correct for the origin of homochirality on earth, if you can prove that those prerequisites existed during the emergence of life," said Jafarpour. "But whether those foundations exist or not, for life that emerged anywhere in the universe, you'd expect that it would have self-replication, and our model says that's enough to get homochirality."
The model relies on mathematical and computational techniques that were not available in Frank's time. It takes into account the chance events involving individual molecules—which chiral self-replicator happens to find its next substrate first. The detailed statistics built into the model reveal that if self-replication is occurring efficiently enough, this incidental advantage can grow into dominance of one chirality over the other. The forerunner of this mathematical mechanism came from Biancalani's previous work on how chance events influence the foraging patterns of ant colonies.
Goldenfeld attributes part of their success to the interdisciplinary environment of the IGB and of the Institute for Universal Biology (IUB), a member of the NASA Astrobiology Institute. "If we hadn't been in this environment, we wouldn't have been so prepared to think about this problem; we might have just stuck with ants, and never made the jump to realizing that we can apply this to this origin of life problem," he said.
The work leads to a key conclusion: since homochirality depends only on the basic principles of life, it is expected to appear wherever life emerges, regardless of the surrounding conditions.
"For me, the most exciting thing is that this mechanism shows that homochirality is really a biosignature of life, a 100% signature, and should be expected anywhere life emerges," said Goldenfeld. "So for example, we just learned that there is a global ocean of liquid water under the ice of Enceladus ... I think that looking for homochirality in the organic molecules that have been detected there would be a fantastic way to look for life there."
More information: Farshid Jafarpour et al. Noise-Induced Mechanism for Biological Homochirality of Early Life Self-Replicators, Physical Review Letters (2015). DOI: 10.1103/PhysRevLett.115.158101
Journal information: Physical Review Letters
Provided by University of Illinois at Urbana-Champaign