Scientists find possible replacement for platinum as catalyst
Rice University chemists who developed a unique form of graphene have found a way to embed metallic nanoparticles that turn the material into a useful catalyst for fuel cells and other applications.
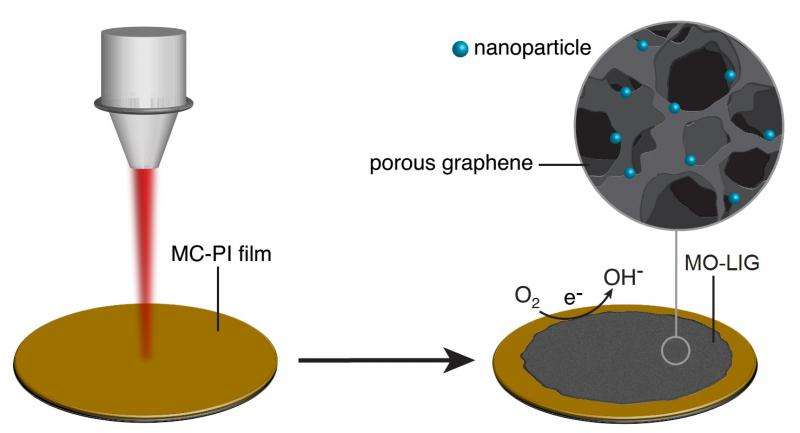
Laser-induced graphene, created by the Rice lab of chemist James Tour last year, is a flexible film with a surface of porous graphene made by exposing a common plastic known as polyimide to a commercial laser-scribing beam. The researchers have now found a way to enhance the product with reactive metals.
The research appears this month in the American Chemical Society journal ACS Nano.
With the discovery, the material that the researchers call "metal oxide-laser induced graphene" (MO-LIG) becomes a new candidate to replace expensive metals like platinum in catalytic fuel-cell applications in which oxygen and hydrogen are converted to water and electricity.
"The wonderful thing about this process is that we can use commercial polymers, with simple inexpensive metal salts added," Tour said. "We then subject them to the commercial laser scriber, which generates metal nanoparticles embedded in graphene. So much of the chemistry is done by the laser, which generates graphene in the open air at room temperature.
"These composites, which have less than 1 percent metal, respond as 'super catalysts' for fuel-cell applications. Other methods to do this take far more steps and require expensive metals and expensive carbon precursors."
Initially, the researchers made laser-induced graphene with commercially available polyimide sheets. Later, they infused liquid polyimide with boron to produce laser-induced graphene with a greatly increased capacity to store an electrical charge, which made it an effective supercapacitor.
For the latest iteration, they mixed the liquid and one of three concentrations containing cobalt, iron or molybdenum metal salts. After condensing each mixture into a film, they treated it with an infrared laser and then heated it in argon gas for half an hour at 750 degrees Celsius.
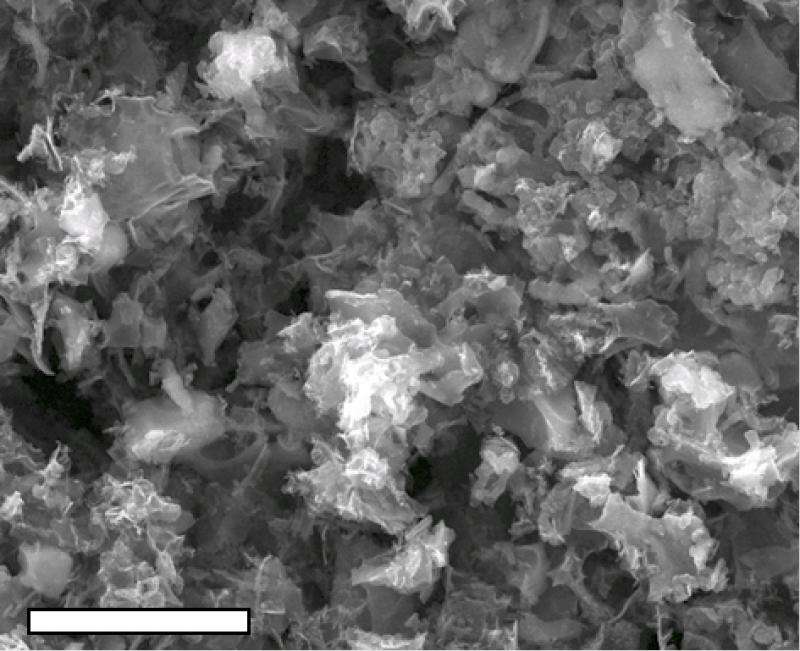
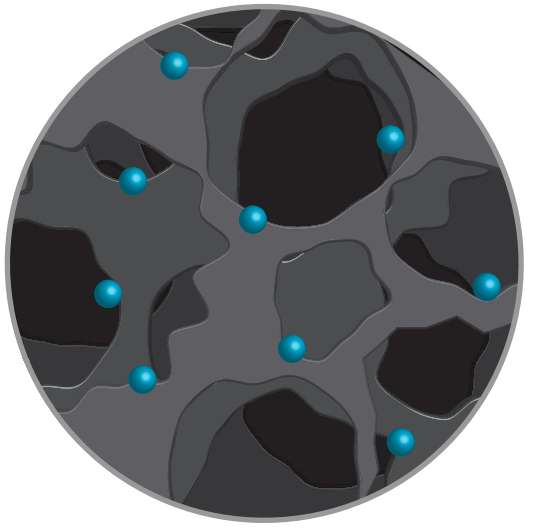
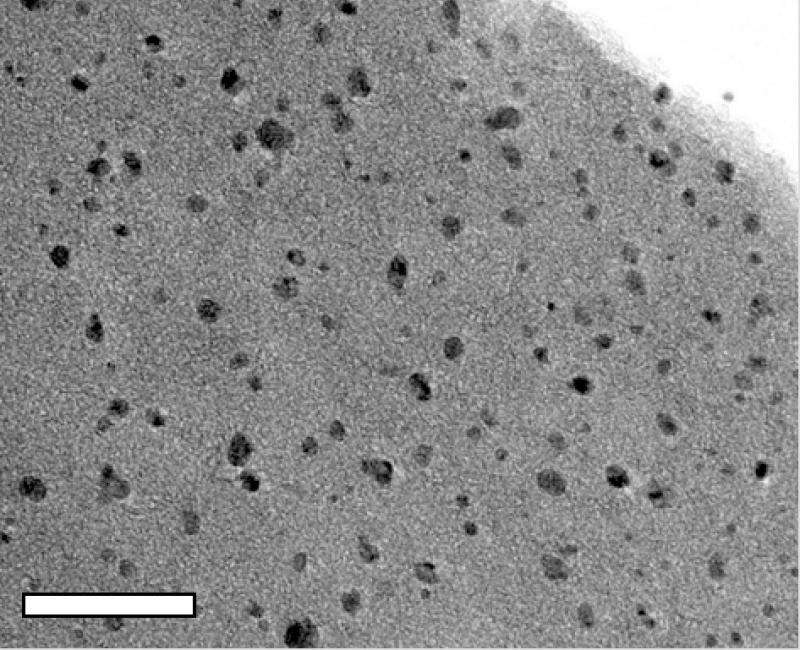
That process produced robust MO-LIGs with metallic, 10-nanometer particles spread evenly through the graphene. Tests showed their ability to catalyze oxygen reduction, an essential chemical reaction in fuel cells. Further doping of the material with sulfur allowed for hydrogen evolution, another catalytic process that converts water into hydrogen, Tour said.
"Remarkably, simple treatment of the graphene-molybdenum oxides with sulfur, which converted the metal oxides to metal sulfides, afforded a hydrogen evolution reaction catalyst, underscoring the broad utility of this approach," he said.
More information: ACS Nano, pubs.acs.org/doi/abs/10.1021/acsnano.5b04138
Journal information: ACS Nano
Provided by Rice University