Highly porous organic polymer shows promise as CO2 trap
(Phys.org) —As the fight against global warming heats up, scientists around the world are in pursuit of ways to generate natural gas without compromising the environment and human health. But what if there were a way to separate and capture carbon dioxide (CO2), a greenhouse gas and major contributor to global warming, before it even had a chance to wreak havoc?
A new material – created by a team of Virginia Commonwealth University scientists – may one day do just that. The team, led by Hani M. El-Kaderi, Ph.D., associate professor of chemistry in the VCU College of Humanities and Sciences, has been examining materials in the laboratory to advance the clean energy initiative.
In a new study published in the Feb. 11 issue of Chemistry of Materials, a journal of the American Chemical Society, El-Kaderi and colleagues report on the synthesis of a highly porous organic polymer that is able to selectively capture CO2 from flue gas and natural gas. The research is highlighted on the journal's cover.
"CO2 capture from the burning of fossil-based fuels has been proposed as a medium-term solution for global warming until new sources of efficient renewable energies and zero emissions (solar and hydrogen) become available at reasonable cost," El-Kaderi said.
"Because our polymers show high capacity and selectivity for CO2 capture, they can be part of the solution and could inspire researchers in this field to adopt similar materials design strategy to mitigating climate change."
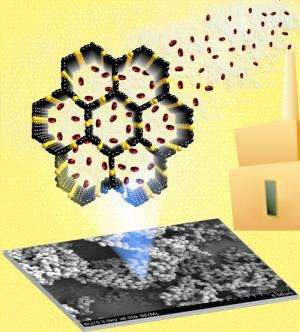
The structure of the polymers created by the VCU team have been physically and chemically engineered at the nano-scale to only trap CO2 when they are exposed to gas mixtures. The polymers are made of extremely small particles that are 500 times smaller than the diameter of a human hair. Each particle has very small pores that are only about one nano-meter, precisely decorated with nitrogen sites to admit and retain CO2 molecules at the exclusion of other gases found in flue gas or natural gas, such as nitrogen and methane. Additionally, the same polymers have also very high surface areas – up to 1,200 square meters per gram – almost six times the area of a tennis court.
"Porous organic polymers are promising candidates for carbon capture and sequestration," said Pezhman Arab, a graduate student in El-Kaderi's research group. "They can be tailor-made using cheap catalysts such as copper to specifically trap harmful gases. They are environment-friendly because of their metal-free nature."
According to El-Kaderi, the team has plans to refine the design of the polymers they have created to enhance its CO2 storage capacity and selective uptake over impurities found in flue gas and methane-rich gases. He said that both parameters are essential for effect adsorbents.
Another research focus will investigate polymers response to light as means for CO2 release.
"In such an energy-saving scenario, CO2 would be released by a mechanical change in the polymer as azo-linkages change their conformation 'dance to light' and squeeze CO2 out of the pores," El-Kaderi said.
More information: "Copper(I)-Catalyzed Synthesis of Nanoporous Azo-Linked Polymers: Impact of Textural Properties on Gas Storage and Selective Carbon Dioxide Capture." Pezhman Arab, Mohammad Gulam Rabbani, Ali Kemal Sekizkardes, Timur İslamoğlu, and Hani M. El-Kaderi. Chemistry of Materials 2014 26 (3), 1385-1392. DOI: 10.1021/cm403161e
Journal information: Chemistry of Materials
Provided by Virginia Commonwealth University




















