Pushing back against drug-resistant bugs
Some pathogens can adapt to the presence of drugs that would normally be lethal, and such antibiotic-resistant microbes are now the scourge of hospitals worldwide. Discovering new antibiotics is a laborious process, but research from a team led by Yi Yan Yang of the A*STAR Institute of Bioengineering and Nanotechnology, Singapore, and James Hedrick of the IBM Almaden Research Center, United States, could breathe new life into existing drugs.
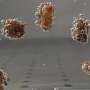
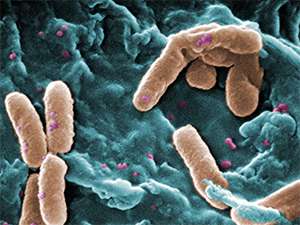
One of the standard escape mechanisms employed by bacteria such as Pseudomonas aeruginosa (see image) entails increased production of proteins that purge or break down drug molecules. Polymers that stick to and disrupt bacterial membranes offer an alternative means for killing resistant cells. However, at elevated concentrations these polymers can also inflict damage on bystander host cells. Yang and Hedrick's team explored whether a combination of polymers and antibiotics might offer a safer alternative that draws on the strengths of both approaches.
They generated two different polymers—which they combined with vitamin E, a standard component of the diet of humans—with chemical properties that enabled them to penetrate cell membranes. Both proved more toxic to microbes than the same polymers without vitamin E, efficiently killing P. aeruginosa as well as two other pathogens, the bacterium Staphylococcus aureus and the fungus Candida albicans. Microscopic analysis indicated that the polymers worked by punching holes in the outer membranes of bacterial and fungal cells. Importantly, none of these compounds proved seriously toxic to rat red blood cells at their effective doses, suggesting that their effects should be relatively specific to their pathogenic targets.
The team subsequently evaluated whether such polymer constructs could bolster antibiotics that tend to lose efficacy against P. aeruginosa. They took the vitamin E-linked polymer formulation that had performed the best against this bacterium and combined it with the antibiotics doxycycline, streptomycin or penicillin G. While all three received a boost from being combined with the polymer, doxycycline showed the greatest promise, proving capable of effectively eradicating bacteria at very low concentrations of both the drug and polymer. This combination also appeared to generate a greater number of holes in the bacterial cell surface, despite doxycycline not normally damaging the outer membrane when acting on its own.
The researchers hypothesize that the two-pronged attack gives doxycycline rapid access to the cellular interior, thus overwhelming the microbe before it has the opportunity to eliminate the drug. "These synergistic combinations may provide a promising way for combating multi-drug resistance," concludes Yang.
More information: Ng, V. W. L., Ke, X., Lee, A. L. Z., Hedrick, J. L. & Yang, Y. Y. "Synergistic co-delivery of membrane-disrupting polymers with commercial antibiotics against highly opportunistic bacteria." Advanced Materials 25, 6730–6736 (2013). dx.doi.org/10.1002/adma.201302952
Journal information: Advanced Materials