Five times less platinum: Fuel cells could become economically more attractive thanks to novel aerogel catalyst

Fuel cells that convert hydrogen into power and only produce pure water as a by-product have the potential to lead individual mobility into an environmentally friendly future. The Paul Scherrer Institute (PSI) has been researching and developing such low-temperature polymer electrolyte fuel cells for more than 10 years and initial field tests have already demonstrated the successful use of these fuel cells in cars and buses. However, further research is still required to improve the durability and economic viability of the technology. An international team of researchers involving the PSI has now manufactured and characterised a novel nanomaterial that could vastly increase the efficiency and shelf-life of these fuel cells – as well as reduce material costs.
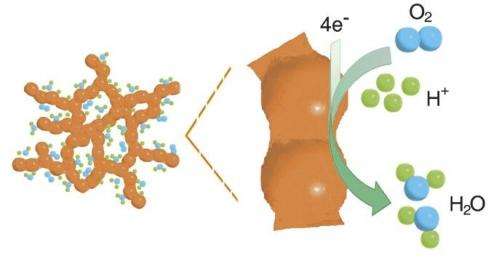
In a hydrogen fuel cell, hydrogen is converted into power and water through electrochemical reactions. A key step in these reactions is the reduction of oxygen at the cell's positive electrode, where oxygen molecules fed into the cell are converted into water. As this reaction takes place very slowly under normal conditions, catalysts are needed to speed up the conversion process. In conventional cells, precious metals such as platinum fullfill this catalytic function. The thin nanoparticles used for this purpose are supported by a substrate typically made of high surface area carbon. However, the carbon substrate can easily become corroded during the common start/stop operation in city traffic or during idling; thereby compromising the function of the catalyst, which in turn shortens the service life of the entire fuel cell. Consequently, researchers have long been looking for catalysts for oxygen reduction that do not need a support and still display a high specific surface area with a large number of catalytic centres as well as good long-term stability.
An international team of researchers involving the PSI has now made considerable progress in this direction. Using a three-dimensional aerogel made of a platinum palladium alloy, they were able to increase the catalytic activity for oxygen reduction at the positive electrode of a hydrogen fuel cell fivefold compared to normal catalysts made of platinum on carbon supports. This means that the same amount of oxygen can now be converted with only a fifth of the amount of precious metals. If this reduction of the necessary platinum load could be transferred onto an industrial scale, it would slash the production costs for these fuel cells. The aerogel, which is a kind of nanostructured foam, has also passed long-term tests in the lab, where the typical operating conditions in a vehicle were simulated.
Light-weight mesh of nanowires
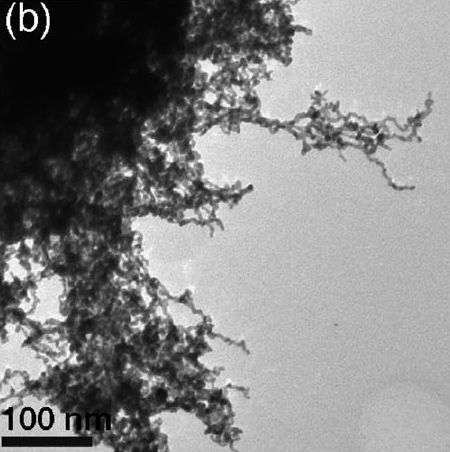
The aerogel now synthesised and characterised by researchers at Dresden University of Technology and at PSI forms a three-dimensional network of nanowires and it features a high porosity and large inner surface. The latter properties facilitate the adsorption of many oxygen molecules onto the catalytically active platinum atoms – a prerequisite for the efficient conversion of oxygen. While catalysts used in commercial fuel cells also exhibit a high degree of porosity and large surfaces, they achieve that only when they consist of platin nanoparticles on a carbon substrate. The key advantage of the new aerogel is that it combines these assets with an extensive three-dimensional structure, which means there is no need for a support whatsoever.
Bimetal alloy aerogel synthesised for the first time
Because of its excellent properties for many applications in electrochemistry and sensing applications, aerogels have attracted a lot of attention in recent years. Numerous teams of researchers all over the world have been chancing their arm at producing new aerogels, mostly safe in the knowledge that useful applications will follow. Until now, however, their success has been limited to a small group of chemical substances: most aerogels are made of oxides or single metals. That said, theoretical considerations had suggested that catalysts made of particular metal alloys would display greater catalytic activity and stability and this had sparked attempts to implement those features in an aerogel catalyst. Synthesising a bimetal aerogel, however, has proven to be easier said than done. "This is the first time that an aerogel made of a metal alloy has ever been synthesised" says Thomas Justus Schmidt, head of the Electrochemistry Laboratory at the PSI and co-author of the study.
The new results confirm the high hopes for these materials. The key to improving the activity of the new aerogel, for instance, is that the alloy with palladium optimises the bond strength between the platinum atoms and the oxygen-containing species. In other words, the bond is so strong that the oxygen molecules remain adsorbed just long enough for the conversion into water but not too strong as to induce the formation of oxides on the catalyst's surface. The fact that the conversion to water is more favourable than the formation of oxides optimises at each point in time the number of available catalytic centres which in turn leads to oxygen molecules being adsorbed and converted at a considerably high rate.
Some questions still unanswered
The researchers are yet to understand another advantage of the alloy, namely the greater stability of the bimetal alloy aerogel compared to monometal aerogels made of pure platinum. "Obviously, the presence of palladium in the aerogel plays a key role here, too, but we don't know yet exactly what impact this has on the stability of the catalyst," explains T.J. Schmidt. The scientists would now like to spend the next three years focusing on this and other questions regarding the new nanomaterial in a follow-up project. "We have just finished the draft for a funding application together with Dresden University of Technology to give the project we have been funding internally up to now a broader financial footing."
More information: Liu, W. et al. Bimetall-Aerogele: hoch effiziente Elektrokatalysatoren für die Sauerstoffreduktion, Angewandte Chemie. DOI: 10.1002/ange.201303109
Journal information: Angewandte Chemie
Provided by Paul Scherrer Institute