Predictive models of environmental reaction kinetics made more accurate, scalable
Predictive models of biogeochemical interactions in soils are more accurate and scalable if they consider the reaction chemistry that occurs in distinct soil pore structures, or domains. These findings are the result of integrated computational and experimental pore-scale reaction kinetics studies conducted by a team of EMSL scientists and users.
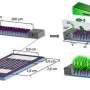
For their studies, the team built a silicon micromodel (8.1 mm × 4.5 mm × 0.028 mm) with a pore-scale structure mimicking that found in nature and coated it with a thin film of the iron oxide hematite (Fe2O3). Iron oxides play a key role in electron exchanges that occur in soil among minerals and microbes. This exchange affects microbial respiration as well as the solubility and, thus, mobility of metals—an especially important consideration in the case of contaminants, such as radionuclides. The team injected the hematite-coated micromodel with reduced flavin mononucleotide (FMNH2), a form of vitamin B2 and effective agent of electron transfer used by microbes, in solutions of varying acidity.
As the reduced FMNH2 gave electrons to and dissolved the hematite, the team studied hematite dissolution both in situ and in real time by using spectroscopy and microscopy tools as well as by measuring the concentration of iron in solution. The hematite reaction kinetics were distinctly different in three domains: (1) an advection domain consisting of a large pore, where fluid flows with relative freedom; (2) a macropore domain, where diffusion dominates but that is well connected to the advection domain; and (3) a micropore domain, where fluid is stagnant and resides in soil aggregates. Compared to a traditional model, which uses one overall reaction kinetic value, the three-domain reaction kinetics system more closely represents real-world conditions. Moreover, multi-domain models enable more accurate scaling of reaction kinetics from the pore scale to the field scale. Such models, with their accuracy and scalability, will be more effective at predicting the environmental impact of geochemical and microbial activities in soil and can help design improved remediation strategies.
More information: Zhang C, C Liu, and Z Shi. 2013. Micromodel Investigation of Transport Effect on the Kinetics of Reductive Dissolution of Hematite, Environmental Science & Technology 47(9):4131–4139. DOI: 10.1021/es304006w
Journal information: Environmental Science & Technology
Provided by Environmental Molecular Sciences Laboratory