Biologists simulate a cell in action
(Phys.org) —The inner workings of a cell involve hundreds of thousands of discrete molecules, engaged in a repeating cycle of interactions that sustain life.
Underlying this activity is the formation of protein. Since they are the building blocks of cellular function, scientists are intensely interested in how cells create protein, said University of Pennsylvania professor Joshua B. Plotkin.
"Protein translation is fundamentally important to all living cells. It's the mechanism by which genetic information is realized into physical entities that actually do things for the cell, allowing it to sense the environment and grow," the biologist said.
Plotkin and postdoctoral fellow Premal Shah in the School of Arts and Sciences have shed new light on what sets the pace of protein translation in cells, using a computational model.
Their discoveries were released in a recent Cell paper titled "Rate-limiting steps in yeast protein translation."
Collaborating with scientists in the United Kingdom, the Penn-led team created a theoretical model of protein translation in the well-studied yeast cell. It simulates translation based on parameters inferred from an experimental snapshot of ribosome interactions with messenger RNAs. Known as mRNAs, these molecules contain the genetic instructions to assemble a protein.
The team discovered that the speed of protein production is limited by the availability of free ribosomes, the cell's protein synthesizing factories.
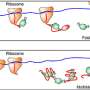
A key part of translation starts with mRNAs. As the ribosome moves along an mRNA string, decoding its genetic information, it forms a mechanical assembly line that spits out a chain of amino acids in the sequence specified by the mRNA.
The ribosome obtains the amino acids from other parts of the cell. Transfer RNAs, known as tRNAs, ferry them over to the translating ribosome. The job of tRNA is to link the mRNA code to the corresponding amino acids. The sequential arrangement of the amino acids determines the type of protein constructed. At the end of the process, the protein is released from the ribosome to begin its job in the cell.
The behavior of ribosomes, transfer RNA and messenger RNA can be compared to a group of people trying to reach a higher floor using an elevator.
The question is what delays the time it takes for everyone to reach a higher floor – the time it takes people to find the elevator or the speed of the elevator itself? If the elevator moves very slowly, we expect to find a large queue of people waiting at its base and more people on the elevator. However, if the elevator moves rapidly, then the main delay is simply the time it takes people to find the elevator in the first place.
Similarly, in a cell, the question is what delays the rate at which ribosomes decode the information in the mRNAs.
Is the delay caused by how often ribosomes bind to the start of mRNA sequences, a process known in biology as initiation, or is it caused by the rate at which the ribosomes then move along the string of mRNA, known as elongation? (Initiation can be compared to the rate at which people step onto the elevator. Elongation is similar to the speed at which the elevator moves between floors.)
It turns out that the answer depends on the cell's environment, and consequently, its stress level.
Under normal circumstances, ribosomes are in short supply, and so the main bottleneck is the initiation step. And Initiation rates are even slower on long mRNAs, the scientists concluded.
This discovery helps resolve an active debate about whether initiation or elongation is the factor that sets the pace of protein production in a cell.
"We show that for normal genes in healthy cells, the rate of initiation by free ribosomes is setting the pace of translation," Plotkin said. "This makes a lot of evolutionary sense. Ribosomes are more costly to produce than tRNA's. And so it's better for the cell to be conservative and have slightly fewer ribosomes and a slight overabundance of tRNAs" he said.
That's the case for healthy cells. Circumstances change when the cells are stressed.
The rate of protein translation in that case is set by elongation, the rate at which ribosomes can process a string of mRNA. That's because a stressed cell is depleted of amino acids, and so has fewer tRNAs available for elongation. The ribosome has to wait longer for a tRNA to bring the corresponding amino acids to each section of mRNA.
The researchers found that stressed cells can cope by putting the protein factory on standby.
"By scaling back the initiation rate under stress, a cell lowers its expectations and actually gets relatively more protein yield. You can compare it to not trying to drive 60 miles an hour if you don't have enough gas, so you can get to where you're going," Plotkin said.
Whether initiation or elongation sets the pace of protein translation depends on the cell's environment, researchers found.
Knowing how the process works is helpful in a variety of biotechnology applications, they said. For example, scientists could find ways to optimize a cell's protein production when it comes to beneficial proteins such as insulin.
Showing how all the different parts of translation work together in concert meant the scientists' model had to be complex.
"The challenge was trying to optimize the computer code and get so many different variables to work together. There are 3 million tRNAs, 200,000 ribosomes and 60,000 mRNAs in a cell. We keep track of all these molecules in real time. Coming up with ways to minimize the time it would take to run these efficiently was a computational challenge," said Shah, who wrote the computer model.
Plotkin said the computer model allowed researchers to perform tests that would take decades in a lab setting.
"Instead, it took us a month of running simulations. We systematically varied the abundance of molecules in a cell, examined every stress condition, and found all the responses," he said.
The model wasn't perfect. The researchers had to omit a host of details found in real cells, such as the time it takes for tRNA to get a new amino acid.
"But we argue that we retain enough essential aspects of protein translation," Plotkin said. "Computational power has reached such a scale that we can study the main processes of life using a computer."
Eventually, a computer model will inform experiments and allow empirical biologists to know which experiment will be more elucidating, he said.
And technology has reached a point that biologists can now start to answer questions that are nearly impossible to resolve in a traditional lab setting.
More information: www.cell.com/abstract/S0092-8674%2813%2900655-7
Journal information: Cell
Provided by University of Pennsylvania