Biologists discover highly complex communication system in aquatic cyanobacteria
Land plants can "see," but can microscopic plants see better? New research from Indiana University has uncovered a give-and-take communication system between and within photoreceptors in freshwater-dwelling cyanobacteria that works at a level of complexity beyond those seen in plants or other organisms.
The new work by IU Bloomington biologist David M. Kehoe and Ph.D. student Adam N. Bussell—published in the Proceedings of the National Academy of Sciences—not only identifies a new type of photoreceptor that can sense four different light colors at once, but for the first time demonstrates the regulation of the abundance of one photoreceptor by a second in cyanobacteria.
Kehoe, a professor in the College of Arts and Sciences' Department of Biology, said identification of both hierarchical control of one photoreceptor over another and of a complex communication system within a single photoreceptor that is simultaneously integrating information about four different light colors opens a new chapter in understanding cyanobacteria complexity and how their photoreceptors work.
Cyanobacteria are of tremendous evolutionary and ecological importance because the 3 billion-year-old aquatic bacteria, historically known as "blue-green algae," were the first group of organisms on Earth to release oxygen during photosynthesis. Their photosynthetic activity currently accounts for about 40 percent of our planet's oxygen production.
In analyzing the newly discovered photoreceptor, called IflA (influenced by far-red light), and its ability to use two distinct photosensory domains to respond to blue, green, red and far-red light, Kehoe and Bussell believe they may have uncovered unique and advantageous machinery for life in aquatic environments that are not needed on land.
"Unlike air, water absorbs far-red light very well, red light well, green light moderately and blue light poorly," Bussell said. "So aquatic cyanobacteria face big changes in the relative amounts of these colors just by moving up or down a few meters in the water column. These are not conditions that land plants have to face."
Living in a world where light color ratios and irradiance levels vary greatly at different depths, and where additional information about depth, competing photosynthesizers, time of day and other parameters can affect survival, the benefits of a complex communication system may be essential. In this case, it's the ability of the two IflA photoreceptor domains—the portion of a protein's sequence with a discrete structure and function that is independent of the rest of the protein chain—to affect each other's behavior during light sensing.
"Our results show that these domains are interacting with each other and integrating information about four different colors of light simultaneously," Kehoe said. "This kind of complexity has never been found in plants or other organisms. Although plants are known to integrate blue light information with red and far-red light information, multiple photoreceptors are used for this, and the information is integrated downstream of the photoreceptors, during signal transduction."
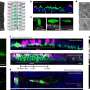
Specifically, the researchers isolated the IflA gene from cells of the filamentous, freshwater cyanobacterium Fremyella diplosiphon and then overexpressed various regions of the IflA protein in the bacteria Escherichia coli that had been engineered to produce the light-sensing chromophores needed by IflA for photoperception. They found the three amino acids that needed to bind the two chromophores used by IflA and that one protein domain sensed red and far-red light, while the other sensed blue and green light. Mutating these amino acids alone and in combination led to the creation of seven different colored forms of IflA when each was overexpressed in E. coli cells (see photo).
Adding to the complexity of this system is the first description, in bacteria, of the strong regulation of the abundance of IflA by a second photoreceptor called RcaE, which senses red and green light and represses the amount of IflA in red light.
"The RcaE-mediated increase in IflA abundance as the ratio of green to red light increases resulted in IflA being about seven or eight times more abundant in green light than in red light," Kehoe said. "This has not ever been seen in prokaryotic phytochrome family members and demonstrates that complex interactions exist between these photoreceptors as well."
More information: www.pnas.org/content/early/201 … /1303371110.abstract
Journal information: Proceedings of the National Academy of Sciences
Provided by Indiana University