Scanning electrochemical microscopy decisively optimised: Researchers measure oxygen consumption of individual cells
How active a living cell is can be seen by its oxygen consumption. The method for determining this consumption has now been significantly improved by chemists in Bochum. The problem up to now was that the measuring electrode altered the oxygen consumption in the cell's environment much more than the cell itself. "We already found that out twelve years ago," says Prof. Dr. Wolfgang Schuhmann from the Department of Analytical Chemistry at the Ruhr-Universität. "Now we have finally managed to make the measuring electrode an spectator." Together with his team, he reports in the "International Edition" of the journal Angewandte Chemie.
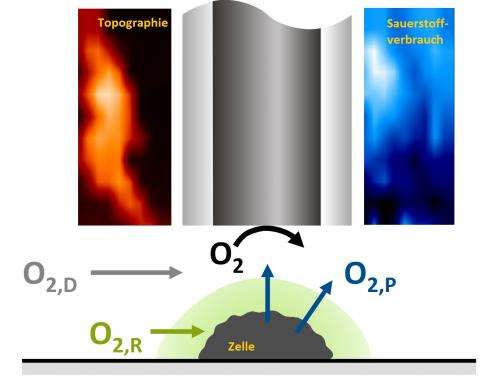
Cells need oxygen for various metabolic processes, for example to break down glucose. To measure its consumption, researchers have to detect very small signals in a large background noise. For this they use scanning electrochemical microscopy, for which they need to position electrodes with a diameter of five micrometres or below at a distance of 200 nanometres from the cell. To this end, the RUB team has developed a special process over the last few years, with which the distance of the electrode to the cell can be precisely controlled.
Using the electrode, the researchers first generate oxygen in the aqueous environment of the cell, and then they measure how much of this oxygen the cell utilises. For this purpose, they give the electrode a certain potential at the beginning. This has the effect that electrons are extracted from water in the cell environment under formation of oxygen. The cell can use the oxygen for its metabolism; however, at the same time, the microelectrode applied by the researchers competes against it. They change the potential at the electrode so that the reaction reverses: oxygen is now converted to water. The scientists use the electrode to measure the electrons flowing and thus obtain a measure of the oxygen consumption in the local environment. The more oxygen the cell uses for its metabolism, the less oxygen is left for the current-generating reaction at the electrode. Thus, the lower the current flow measured, the greater the activity of the cell. This method is termed the redox competition mode.
In the methods used so far, the oxygen consumption caused by the electrode was significantly higher than that of the cell. "The measurement itself thus caused a stronger local change in the oxygen concentration than the cell metabolism," explains Prof. Schuhmann. It was essential to measure the activity of the cell very quickly after the oxygen was generated at the microelectrode, i.e. after twenty milliseconds. If you wait longer, the electrode deprives the cell of oxygen instead of using the oxygen from the environment that the researchers had artificially created in advance. Three factors were therefore crucial for the success of the Bochum method: the highly accurate position of the electrodes, the redox competition mode and the rapid measuring time.
More information: Nebel, M. et al. (2013): Visualization of oxygen consumption of single living cells by scanning electrochemical microscopy: the influence of the faradaic tip reaction, Angewandte Chemie International Edition, DOI: 10.1002/anie.201301098
Journal information: Angewandte Chemie , Angewandte Chemie International Edition
Provided by Ruhr-Universitaet-Bochum