Picking apart photosynthesis: New insights could lead to better catalysts for water splitting
(Phys.org) —Chemists at the California Institute of Technology (Caltech) and the Lawrence Berkeley National Laboratory believe they can now explain one of the remaining mysteries of photosynthesis, the chemical process by which plants convert sunlight into usable energy and generate the oxygen that we breathe. The finding suggests a new way of approaching the design of catalysts that drive the water-splitting reactions of artificial photosynthesis.
"If we want to make systems that can do artificial photosynthesis, it's important that we understand how the system found in nature functions," says Theodor Agapie, an assistant professor of chemistry at Caltech and principal investigator on a paper in the journal Nature Chemistry that describes the new results.
One of the key pieces of biological machinery that enables photosynthesis is a conglomeration of proteins and pigments known as photosystem II. Within that system lies a small cluster of atoms, called the oxygen-evolving complex, where water molecules are split and molecular oxygen is made. Although this oxygen-producing process has been studied extensively, the role that various parts of the cluster play has remained unclear.
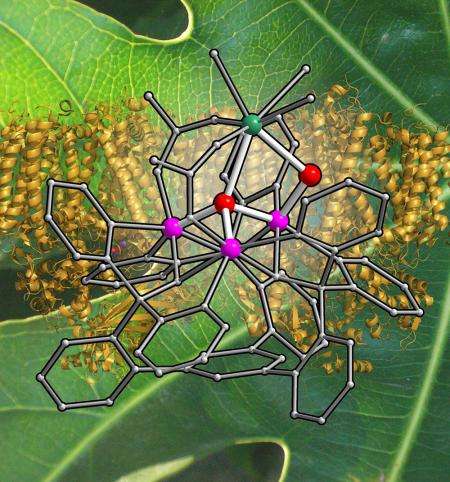
The oxygen-evolving complex performs a reaction that requires the transfer of electrons, making it an example of what is known as a redox, or oxidation-reduction, reaction. The cluster can be described as a "mixed-metal cluster" because in addition to oxygen, it includes two types of metals—one that is redox active, or capable of participating in the transfer of electrons (in this case, manganese), and one that is redox inactive (calcium).
"Since calcium is redox inactive, people have long wondered what role it might play in this cluster," Agapie says.
It has been difficult to solve that mystery in large part because the oxygen-evolving complex is just a cog in the much larger machine that is photosystem II; it is hard to study the smaller piece because there is so much going on with the whole. To get around this, Agapie's graduate student Emily Tsui prepared a series of compounds that are structurally related to the oxygen-evolving complex. She built upon an organic scaffold in a stepwise fashion, first adding three manganese centers and then attaching a fourth metal. By varying that fourth metal to be calcium and then different redox-inactive metals, such as strontium, sodium, yttrium, and zinc, Tsui was able to compare the effects of the metals on the chemical properties of the compound.
"When making mixed-metal clusters, researchers usually mix simple chemical precursors and hope the metals will self-assemble in desired structures," Tsui says. "That makes it hard to control the product. By preparing these clusters in a much more methodical way, we've been able to get just the right structures."
It turns out that the redox-inactive metals affect the way electrons are transferred in such systems. To make molecular oxygen, the manganese atoms must activate the oxygen atoms connected to the metals in the complex. In order to do that, the manganese atoms must first transfer away several electrons. Redox-inactive metals that tug more strongly on the electrons of the oxygen atoms make it more difficult for manganese to do this. But calcium does not draw electrons strongly toward itself. Therefore, it allows the manganese atoms to transfer away electrons and activate the oxygen atoms that go on to make molecular oxygen.
A number of the catalysts that are currently being developed to drive artificial photosynthesis are mixed-metal oxide catalysts. It has again been unclear what role the redox-inactive metals in these mixed catalysts play. The new findings suggest that the redox-inactive metals affect the way the electrons are transferred. "If you pick the right redox-inactive metal, you can tune the reduction potential to bring the reaction to the range where it is favorable," Agapie says. "That means we now have a more rational way of thinking about how to design these sorts of catalysts because we know how much the redox-inactive metal affects the redox chemistry."
The paper in Nature Chemistry is titled "Redox-inactive metals modulate the reduction potential in heterometallic manganese-oxido clusters."
Journal information: Nature Chemistry
Provided by California Institute of Technology