New technique developed to separate complex molecular mixtures
Chemists at the University of Liverpool have created a new technique that could be used in industry to separate complex organic chemical mixtures.
Chemical feedstocks containing benzene are used extensively in industry to create modern materials and polymers.
Distillation techniques
Their use relies heavily on distillation techniques which separate complex mixtures into more simple molecules used as building blocks to develop drugs, plastics and new materials. These distillation techniques can be expensive and involve large amounts of energy for hard-to-separate mixtures.
A team of researchers at the University's Department of Chemistry, led by Professor Andrew Cooper, have created organic molecular crystals that are able to separate important organic aromatic molecules by their molecular shape.
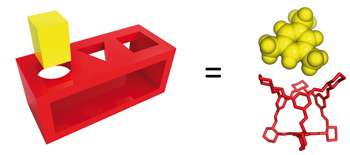
Professor Cooper said: "We were able to demonstrate this new molecule separation technique by synthesising porous organic cage molecules that are highly similar in shape to the molecules that need to be separated.
"Flexibility and motion"
"The holes in these cage molecules act like a shape-selective molecular sieve, rather like a children's wooden shape puzzle. Using computer simulations we revealed how the porous cages separate the aromatic feedstocks and show that, unlike a wooden shape puzzle, the mechanism actually involves flexibility and motion in the cage sieves. "
The ability to separate complex molecules using less energy will be important in the future for current petrochemical and chemical industries and for producing any next-generation sustainable bio-derived chemicals.
The findings are part of a five-year research programme in new materials discovery, and are published in Nature Chemistry.
More information: www.nature.com/nchem/journal/v … full/nchem.1550.html
Journal information: Nature Chemistry
Provided by University of Liverpool