Protein regulates protein folding in cells during stress
(Phys.org)—Cornell researchers have discovered that a protein known for moving cells around in the body also helps regulate a cellular organelle responsible for generating one-third of all proteins in the human body.
The protein, called non-muscle myosin IIB (NMIIB), is required to alleviate stress that occurs when the cell's protein factory, the endoplasmic reticulum, is overburdened.
In the study, published in the Dec. 11 issue of the journal Developmental Cell, the researchers knocked out the gene that codes for NMIIB in mouse cells as well as a model organism, the roundworm C. elegans, and found that when the endoplasmic reticulum was under stress, the cells were unable to respond properly and errors in protein folding were left uncorrected. As a protein's final structure is key to its proper function, improperly folded proteins lead to cell death and underlie the development of human diseases including diabetes, cystic fibrosis, and neurodegenerative and other conformational diseases.
"If cells cannot adjust folding capacity in response to cellular needs, then they die," said Ling Qi, Cornell assistant professor of nutritional sciences and the study's senior author. Yin He, a graduate student in the Qi lab, is the paper's lead author.
When the endoplasmic reticulum is stressed, order is partly restored by a protein called IRE1α, which has been used by organisms throughout evolution. IRE1α senses mis-folded proteins, binds to them and triggers a cascade of signals to the cell's nucleus. The nucleus then responds by improving the folding environment within the endoplasmic reticulum.
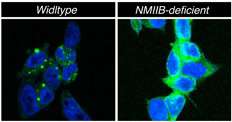
During normal function, NMIIB lies in a folded, inactive form, but during endoplasmic reticulum stress, NMIIB unfolds. When unfurled, NMIIB has a tail that acts as a cantilever, attaching to IRE1α and moving it into aggregates or foci, required for optimal IRE1 activation and function.
"When we knock out myosin, we don't see the IRE1α foci, and if there is no foci, then the downstream signaling and the stress response is attenuated," said Qi.
NMIIB is a cytoskeletal protein, a structural element that exists in the cell's inner fluid and helps provide the cell with its structure. The researchers were surprised to find such a protein involved in IRE1α activation since activation signals during stress were previously thought emanate from compartments of the endoplasmic reticulum, called lumen, where protein folding occurs, Qi added.
"No one has previously reported a link between IRE1α and NMIIB," said He. "Since endoplasmic reticulum stress is associated with so many human diseases, we want to identify novel regulators of these pathways so we can target them therapeutically," she added.
Journal information: Developmental Cell
Provided by Cornell University