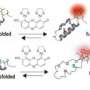
Scientists watch proteins self-assemble
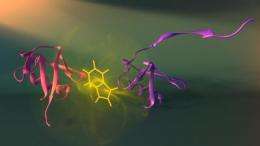
Enabling bioengineers to design new molecular machines for nanotechnology applications is one of the possible outcomes of a study by University of Montreal researchers that was published in Nature Structural and Molecular Biology today.
The scientists have developed a new approach to visualize how proteins assemble, which may also significantly aid our understanding of diseases such as Alzheimer's and Parkinson's, which are caused by errors in assembly.
"In order to survive, all creatures, from bacteria to humans, monitor and transform their environments using small protein nanomachines made of thousands of atoms," explained the senior author of the study, Prof. Stephen Michnick of the university's department of biochemistry. "For example, in our sinuses, there are complex receptor proteins that are activated in the presence of different odor molecules. Some of those scents warn us of danger; others tell us that food is nearby." Proteins are made of long linear chains of amino acids, which have evolved over millions of years to self-assemble extremely rapidly – often within thousandths of a split second - into a working nanomachine. "One of the main challenges for biochemists is to understand how these linear chains assemble into their correct structure given an astronomically large number of other possible forms," Michnick said.
"To understand how a protein goes from a linear chain to a unique assembled structure, we need to capture snapshots of its shape at each stage of assembly said Dr. Alexis Vallée-Bélisle, first author of the study. "The problem is that each step exists for a fleetingly short time and no available technique enables us to obtain precise structural information on these states within such a small time frame. We developed a strategy to monitor protein assembly by integrating fluorescent probes throughout the linear protein chain so that we could detect the structure of each stage of protein assembly, step by step to its final structure." The protein assembly process is not the end of its journey, as a protein can change, through chemical modifications or with age, to take on different forms and functions. "Understanding how a protein goes from being one thing to becoming another is the first step towards understanding and designing protein nanomachines for biotechnologies such as medical and environmental diagnostic sensors, drug synthesis of delivery," Vallée-Bélisle said.
More information: "Visualizing transient protein folding intermediates by tryptophan scanning mutagenesis," published in Nature Structural & Molecular Biology.
Journal information: Nature Structural and Molecular Biology , Nature Structural & Molecular Biology
Provided by University of Montreal