Actin-ratchet tightens contractile ring that severs budding daughter cells from their yeast mothers
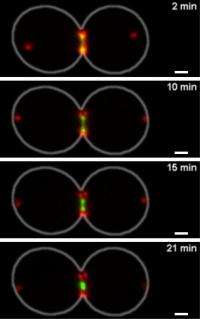
During the final stage of cell division, a short-lived contractile ring constricts the cellular membrane and eventually separates the dividing cell in two. Although this "molecular muscle's" composition, mainly actin and myosin, is similar to its skeletal counterpart, the force-producing mechanism is fundamentally different, report researchers at the Stowers Institute for Medical Research in the June 12, 2012, issue of Developmental Cell.
Combining traditional imaging and genetic approaches with a novel quantitative microscopy model, Stowers Investigator Rong Li, Ph.D., and her colleagues reveal that the depolymerization of actin filaments combined with actin cross-linkers, which act like pawls on a ratchet, and not the sliding myosin motors credited with contracting skeletal muscle, is the main driving force behind the tightening of the actomyosin ring that completes cell division in budding yeast.
Their study breaks new ground in the field of cytokinesis and provides new insight into the contraction mechanisms of actomyosin structures in non-muscle cells, which play an important role in cell division but also many other processes such as cell shape changes, cell adhesion and motility. "It had long been known that the contractile ring is made up of actin and myosin, the same molecules that allow our muscles to contract," explains Stowers Investigator Rong Li, Ph.D., who led the team. "As a result, it was logical to assume that these intracellular structures work the same way."
In muscle cells, the so-called motor domain of myosin binds actin and generates tension through a "power stroke"-mechanism fueled by the energy released from ATP hydrolysis. However, budding yeast genetically engineered to lack the motor domain of myosin suffers only minor ill effects. "The motor region was thought to be critical for the proper function of the contractile ring," says graduate student and co-first author Inês Mendes Pinto, "but these yeast cells were still able to divide fairly unimpeded."
Intrigued by a theoretical study that proposed that contractile force may also be generated by actin depolymerization, Mendes Pinto teamed up with Stowers Research Advisors Boris Rubinstein, Ph.D., a biomathematician, and Jay R. Unruh, Ph.D., who specializes in advanced microscopy and Andrei Kucharavy, MS, a biomathematician from the Ecole Polytechnique, Paris, France, who spent time as a summer intern at the institute. In close collaboration, they developed a quantitative microscopic model to account for the observed properties of the actomyosin ring during budding yeast cytokinesis, which pinches off the budding daughter from the mother cell, and make quantitative predictions that could be tested experimentally.
"The Stowers Institute is unique in that it encourages the intense day-to-day interaction between mathematicians, physicists and biologists that is necessary to build this kind of biologically relevant model," says co-first author Rubinstein.
The theoretical framework that had sparked Mendes Pinto's research project rests on progressively shortening actin filaments held together by actin cross-linkers that act like the pawl on a ratchet tightening the actomyosin ring click-by-click.
By blocking actin depolymerization, the process that causes actin filaments to shorten, with the drug Jasplakinolide, the Stowers researchers were able to establish that actin depolymerization indeed plays an important role in yeast cell division. A yeast strain genetically engineered to lack cofilin 1, the main actin depolymerization factor in yeast, confirmed the contribution of actin depolymerization to ring constriction.
"We are unable to follow individual actin filaments in the cell, however," explains Mendes Pinto, "and had to find an alternative way to dissect the mechanism that links actin depolymerization to contraction." A bottom-up model of ring kinetics based on a set of microscopic elements and their interactions allowed them to do just that.
"The model confirmed that the primary force driving budding yeast cytokinesis results from actin depolymerization," says Li, "while myosin's main role is to facilitate the disassembly of actin." What's more, the model explained another puzzling phenomenon: In any given cell type, the time it takes to complete cell division is the same regardless of the actomyosin ring's initial size.
Journal information: Developmental Cell
Provided by Stowers Institute for Medical Research














